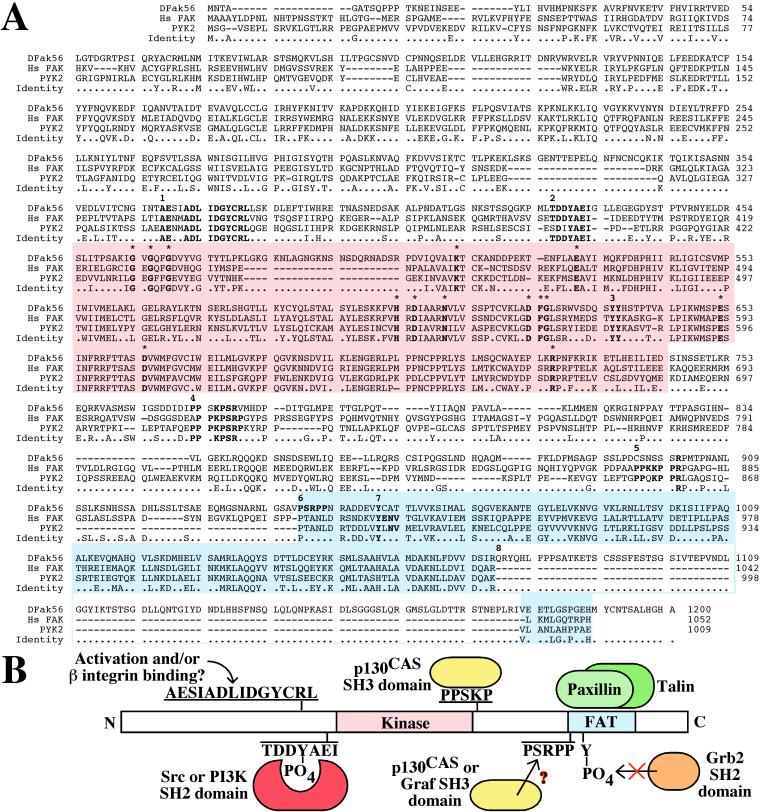
Figure 1.
(A) Alignment of the predicted protein sequence of DFak56 with (human) Hs FAK and PYK2. Positions at which DFak56 is identical to either Hs FAK or PYK2 are indicated on the Identity line. The kinase and focal adhesion targeting (FAT) domains are highlighted in red and blue, respectively. Within the kinase domain, 14 residues invariant among all tyrosine kinases have been indicated by bold characters and asterisks. Additional sequences of interest, also in bold, are distinguished by numbers: 1; the most prominent stretch of identity outside the kinase domain; 2, the autophosphorylation site; 3, a dityrosine motif, located in the activation loop of the kinase domain; 4, a proline-rich sequence required for interaction between FAK and CAS; 5, a second proline-rich sequence that has been implicated in interactions with both CAS and Graf; 6, a proline-rich sequence unique to DFak56; 7, a site of src phosphorylation that mediates an interaction with Grb2; and 8, the 104-aa insertion at the end of the FAT domain of DFak56. (B) Summary of potential DFak56 interactions predicted by homology. It seems highly likely that DFak56 will interact with signaling molecules such as PI3K, src, and p130CAS, and the focal contact proteins talin and paxillin. The interaction with Grb2, on the other hand, does not seem to be conserved, and an interaction with Graf (assuming that there is a Drosophila homolog) seems unlikely.