Figure 5.
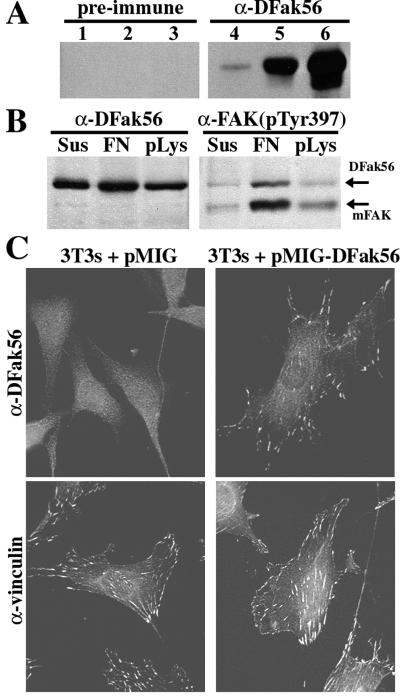
DFak56 functions like endogenous FAK in NIH 3T3 cells. (A) Western blots of 100 μg of embryo extract (lanes 1 and 4), and 33 μg of extract from S2 cells transfected with pCaSpeR-hs (lanes 2 and 5) or S2 cells transfected with pCaSpeR-hs(DFak56) (lanes 3 and 6). Identical blots were probed with antiserum 1563C (α-DFak56) or preimmune serum. 1563C recognizes a 140-kDa band (the predicted size of DFak56) in protein extracts from both embryos and S2 cells whereas the preimmune serum does not. The increase in signal in this band when S2 cells are transfected with pCaSpeR-hs(DFak56) and heat-shocked (compare lanes 5 and 6) demonstrates specificity for DFak56. Similar results were obtained with antiserum 1562C (not shown). (B) Western blots of protein extracts from NIH 3T3 cells infected with pMIG-DFak56, a retroviral expression vector. Equal numbers of infected cells were maintained in suspension (Sus), were plated on fibronectin (FN), or were plated on poly-lysine (pLys) for 2 hr and were lysed. Identical blots were probed with either 1563C (α-DFak56) or a polyclonal antiserum specific to the phosphorylated form of the FAK autophosphorylation site [α-FAK(pTyr397)]. (C) Subcellular localization of DFak56 in NIH 3T3 cells infected with either the empty retroviral vector, pMIG (Left) or pMIG-DFak56 (Right). Cells were allowed to adhere to fibronectin overnight and were fixed and stained with 1563C (α-DFak56) or an antivinculin antiserum.