Abstract
While a knowledge of cercarial infection rates is essential to an understanding of the dynamics of bilharziasis transmission, rather little attention has been paid to methods of determining these rates. The author considers that the most valuable information is likely to be obtained if studies of infectivity are carried out hand in hand with studies of snail population density and structure. Different methods employed in examining snail populations for infection are discussed, together with the patterns of Schistosoma haematobium and S. mansoni cercarial production in relation to natural infections. Other important aspects considered are cercarial shedding in relation to unisexual and bisexual infections, and the use of laboratory animal exposure in natural waters as a means of determining transmission patterns. The author stresses that infection rates and worm burdens obtained from the animal exposure method have to be evaluated carefully in relation to the state of the habitat.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNECKE S., PITCHFORD R. J., JACOBS A. J. Some further observations on bilharziasis in the Transvaal. S Afr Med J. 1955 Apr 2;29(14):314–323. [PubMed] [Google Scholar]
- BARBOSA F. S., OLIVIER L. Studies on the snail vectors of bilharziasis mansoni in North-eastern Brazil. Bull World Health Organ. 1958;18(5-6):895–908. [PMC free article] [PubMed] [Google Scholar]
- CHERNIN E., DUNAVAN C. A. The influence of host-parasite dispersion upon the capacity of Shistosoma mansoni miracidia to infect Australorbis glabratus. Am J Trop Med Hyg. 1962 Jul;11:455–471. doi: 10.4269/ajtmh.1962.11.455. [DOI] [PubMed] [Google Scholar]
- CRIDLAND C. C. Ecological factors affecting the numbers of snails in permanent bodies of water. J Trop Med Hyg. 1957 Oct;60(10):250–256. [PubMed] [Google Scholar]
- HAIRSTON N. G. Suggestions regarding some problems in the evaluation of molluscicides in the field. Bull World Health Organ. 1961;25:731–737. [PMC free article] [PubMed] [Google Scholar]
- MALDONADO J. F. The daily emergence of the cercaria of Schistosoma mansoni. Bol Asoc Med P R. 1959 Sep;51:336–339. [PubMed] [Google Scholar]
- PESIGAN T. P., HAIRSTON N. G., JAUREGUI J. J., GARCIA E. G., SANTOS A. T., SANTOS B. C., BESA A. A. Studies on Schistosoma japonicum infection in the Philippines. 2. The molluscan host. Bull World Health Organ. 1958;18(4):481–578. [PMC free article] [PubMed] [Google Scholar]
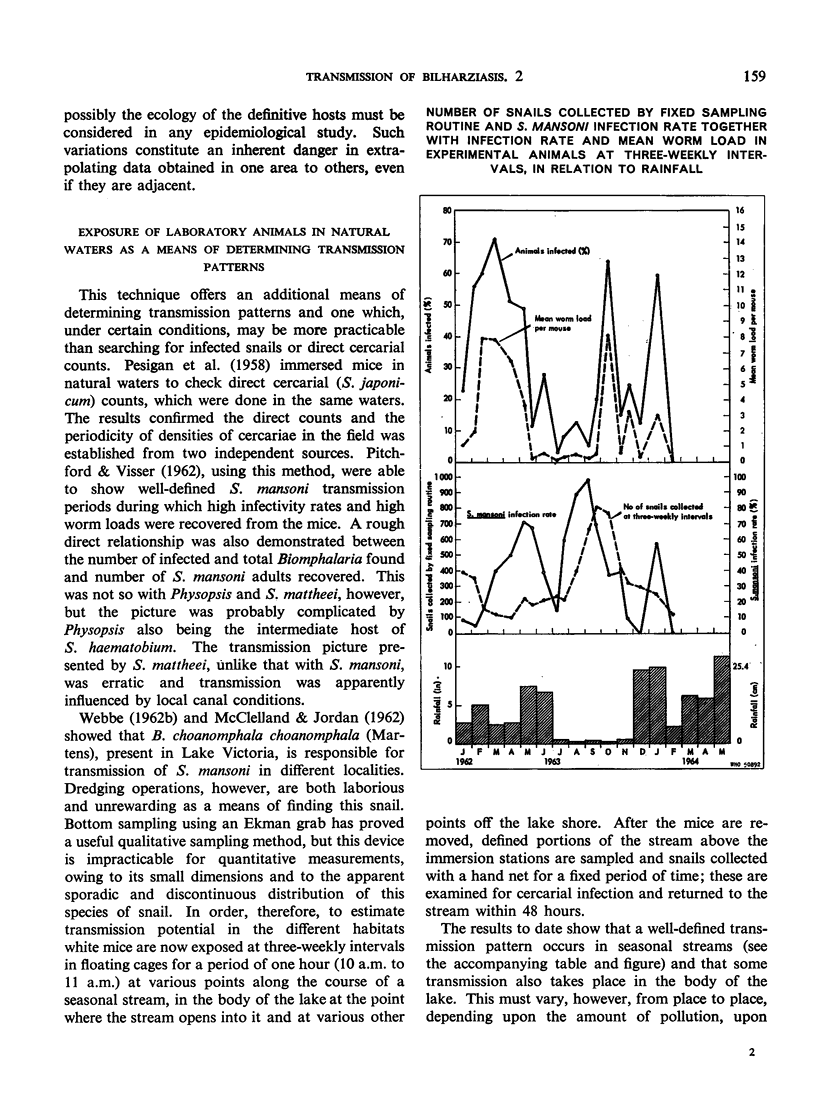
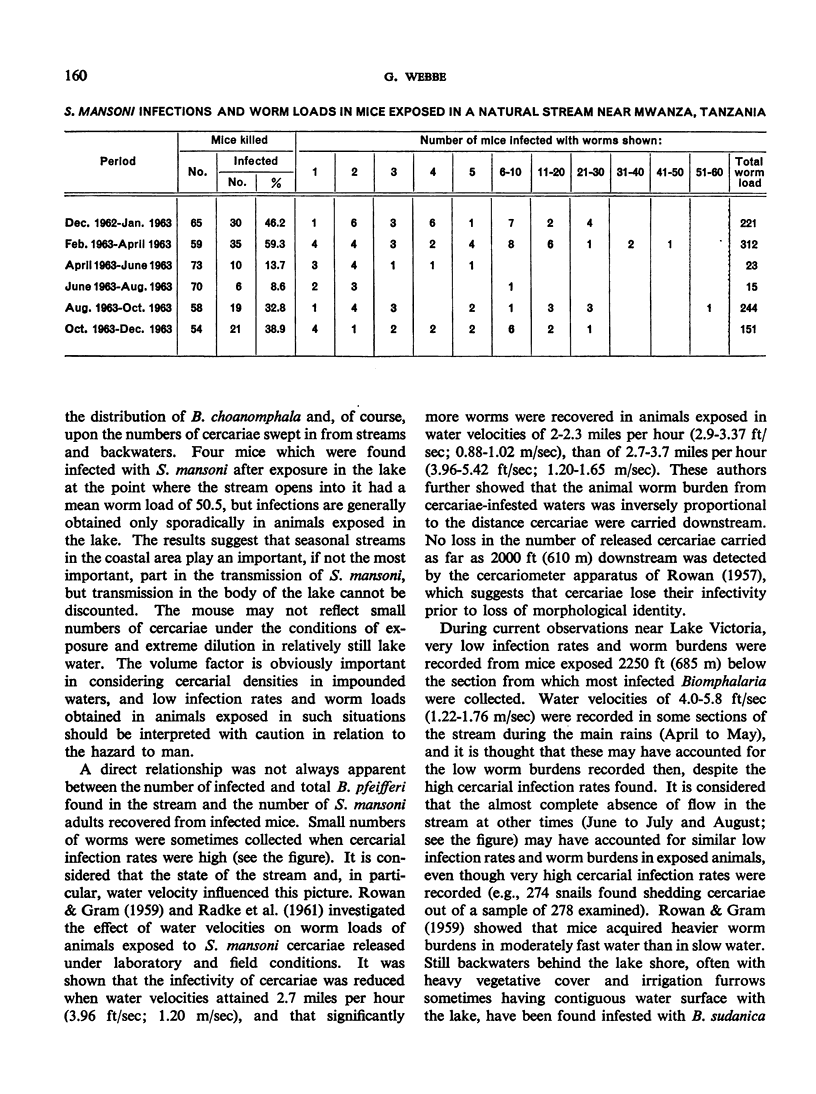
- PITCHFORD R. J., VISSER P. S. Results of exposing mice to schistosomiasis by immersion in natural water. Trans R Soc Trop Med Hyg. 1962 Jul;56:294–301. doi: 10.1016/0035-9203(62)90051-2. [DOI] [PubMed] [Google Scholar]
- RADKE M. G., RITCHIE L. S., ROWAN W. B. Effects of water velocities on worm burdens of animals exposed to Schistosoma mansoni cercariae released under laboratory and field conditions. Exp Parasitol. 1961 Nov;11:323–331. doi: 10.1016/0014-4894(61)90039-x. [DOI] [PubMed] [Google Scholar]
- ROWAN W. B. Daily periodicity of Schistosoma mansoni cercariae in Puerto Rican waters. Am J Trop Med Hyg. 1958 Jul;7(4):374–381. doi: 10.4269/ajtmh.1958.7.374. [DOI] [PubMed] [Google Scholar]
- ROWAN W. B., GRAM A. L. Relation of water velocity to Schistosoma mansoni infection in mice. Am J Trop Med Hyg. 1959 Nov;8:630–634. doi: 10.4269/ajtmh.1959.8.630. [DOI] [PubMed] [Google Scholar]
- WEBBE G., READ W. W. The use of cobalt 60 for labelling snails in the study of field populations. Ann Trop Med Parasitol. 1962 Jul;56:206–209. doi: 10.1080/00034983.1962.11686110. [DOI] [PubMed] [Google Scholar]
- WEBBE G. The transmission of Schistosoma haematobium in an area of Lake Province, Tanganyika. Bull World Health Organ. 1962;27:59–85. [PMC free article] [PubMed] [Google Scholar]
- Webbe G. Transmission of Bilharziasis. 1. Some essential aspects of Snail population dynamics and their study. Bull World Health Organ. 1965;33(2):147–153. [PMC free article] [PubMed] [Google Scholar]


