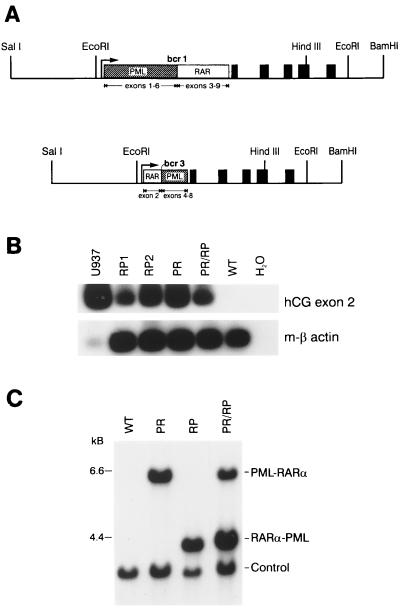
Figure 1.
Construction, expression, and detection of hCG-PML-RARα and hCG RARα-PML transgenes. (A) Diagram of the transgenes. PR and RP cDNAs were ligated into the hCG gene at a synthetic polylinker near the hCG promoter as described previously (2). The 2.5-kb SalI-EcoRI fragment of the transgene contains the 5′ flanking region of the hCG gene. The PML-RARα and RARα-PML cDNAs are shown as boxes and are inserted into the 5′ untranslated region of the hCG gene. The solid boxes represent the five coding exons of hCG. The transgenes also contain the native 3′ flanking sequence of the gene to the BamHI site. (B) RT-PCR analysis of bone marrow RNA derived from PR and RP founder lines and from U937 cells [a human promonocytic cell line that expresses hCG, as a positive control (2)]. Primers spanning hCG exons 1–2 and 3–2 junctions were used to amplify hCG mRNA, as described previously (2). Primers specific for mouse β-actin were used to control for cDNA quality in each sample. The low signal generated in the U937 β-actin lane reflects the specificity of these primers for mouse β-actin. (C) Southern blot detection of transgenes. Tail DNAs were digested with EcoRI and analyzed by Southern blotting. Transgenes were identified with a radiolabeled probe derived from exon 2 of hCG. Doubly transgenic animals contained the expected 6- and 4-kb fragments corresponding to the PR and RP transgenes, respectively. The “control” band is generated by the hybridization of a probe specific for the murine granzyme A gene and serves as a control for DNA loading, transfer, and hybridization.