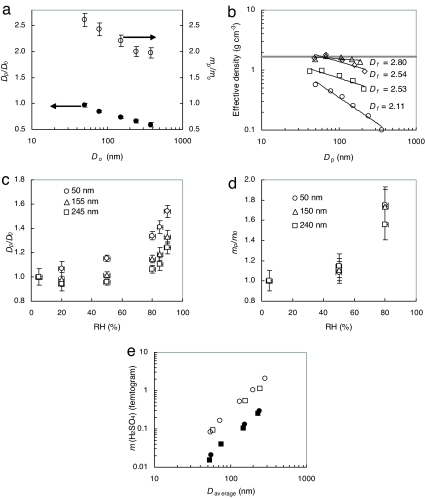
Fig. 2.
Effects of sulfuric acid on properties of aerosol particles. (a) Changes in the particle mobility size and mass after exposure of soot agglomerates to sulfuric acid and water: the particle size Dp/Do (filled circles and left axis) and mass mp/mo (open circles and right axis) ratios after H2SO4 exposure and H2O stabilization at 5% RH. The gaseous concentration of sulfuric acid is 1.4 × 1010 molecules·cm−3. The subscripts p and o refer to H2SO4-coated soot particles at 5% RH and fresh soot particles, respectively. (b) Effective density (ρeff) of fresh and exposed soot determined from the mass [differential mobility analyzer–aerosol particle mass (DMA–APM)] and mobility (DMA–DMA) measurements: circles, fresh soot; diamonds, H2SO4-coated soot at RH 5%; squares, H2SO4-coated soot heated to 200°C to remove condensed sulfuric acid; triangles, H2SO4-coated soot humidified to RH 90% and then dried to a RH of 5%. The gray line corresponds to the estimated bulk density of the soot–H2SO4 mixture (1.7 g·cm−3). The H2SO4 concentration is 1.4 × 1010 molecules·cm−3. The fractal dimension (Df) is 2 for a plane and 3 for a solid sphere. (c) Hygroscopic mobility-size growth ratio (Dp/Do) of H2SO4-coated soot particles. The H2SO4 concentration is 1.4 × 1010 molecules·cm−3. The subscripts p and o refer to H2SO4-coated soot particles at a higher RH and at 5% RH, respectively. The circles, triangles, and squares correspond to coated soot particles with fresh-particle sizes of 50, 155, and 245 nm, respectively. (d) Hygroscopic mass growth ratio (mp/mo) of H2SO4-coated soot particles. The H2SO4 concentration is 1.4 × 1010 molecules·cm−3. The subscripts p and o refer to H2SO4-coated soot particles at a higher RH and at 5% RH, respectively. The circles, triangles, and squares correspond to coated soot particles with fresh-particle sizes of 50, 155, and 245 nm, respectively. (e) Absolute-mass coating of sulfuric acid on soot agglomerates (squares) and PSL spheres (circles) after H2SO4 exposure and H2O stabilization at 5% RH. Open and filled symbols represent sulfuric acid vapor concentrations of 1.4 × 1010 and 2.5 × 109 molecules·cm−3, respectively. The diameter (Daverage) corresponds to the average of uncoated and coated particle, because the mobility diameter varies after sulfuric acid condensation. In a, c, and d, the vertical error bars represent random error of the measurements (2 SDs), and the values are averaged over at least two measurements.