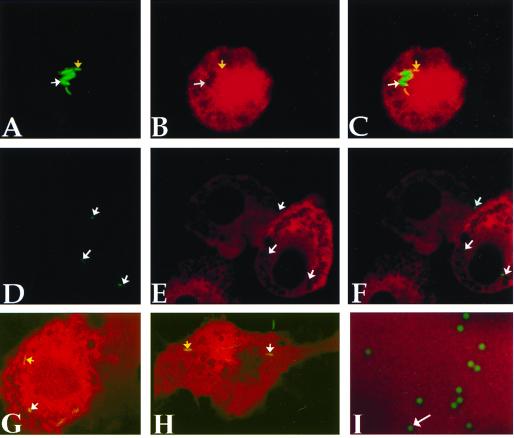
Figure 1.
Mycobacteria reside within permeable phagosomes admitting molecules up to 70 kDa. Macrophages infected with GFP-BCG for 18 hours were microinjected with Texas Red-tagged dextrans (3 kDa) and immediately were visualized by confocal microscopy. A and D show fluorescence in the green channel only, B and E show fluorescence in the red channel only, and C, F, G, and H are merged images. Colocalization of green fluorescent bacilli (A, yellow arrow) with 3-kDa-sized Texas Red-tagged dextrans (B, yellow arrow) manifested as yellow bacilli upon merging of the two channels (C, yellow arrow). Not all GFP-BCG (A, white arrow) are found within membrane-permeable vesicles (B, white arrow), and therefore did not exhibit signal colocalization (C, white arrow). Microinjection of 2,000-kDa-sized dextrans revealed green bacilli (D, white arrow) within vesicles clearly impermeable to the infected dextrans (E and F, white arrows). Mycobacterial phagosomes are permeable to injected proteins, within the appropriate size range, as ovalbumin accesses the mycobacterial phagosome (G, yellow arrow). Molecules of up to 70 kDa in size access the mycobacterial phagosome, as evidenced by the presence of yellow bacilli indicating signal colocalization (H, yellow arrow). Colocalization is not a limit of resolution artefact as Oregon-green-tagged beads ≤0.5 μm are readily resolved from the surrounding Texas-red-tagged dextrans (I, white arrow).