Abstract
Purpose
The purpose of this study was to evaluate the predictive value of 2-deoxy-2-[F-18]fluoro-d-glucose-positron emission tomography (FDG-PET) following concurrent chemoradiotherapy (CRT) on survival in patients with carcinoma of the oropharynx (OPC).
Methods
Eighteen patients with primary OPC who underwent PET pre- and post-CRT were evaluated prospectively for survival. The prognostic performance of post-CRT PET and CT for recurrence was compared.
Results
Patients with positive post-CRT PET exhibited significantly lower 2-year cause-specific survival and disease-free survival (50% vs. 91%, P < 0.05 and 0% vs. 83%, P < 0.0001); however, patients with positive post-CRT CT did not exhibit any significant difference (67% vs. 83%, P = 0.416 and 50% vs. 75%, P = 0.070). Other factors, such as clinical and pre-CRT PET variables, also did not indicate any significant difference. The accuracy of prediction of residual and local recurrence for post-CRT PET and CT (local%/regional%) was 83%/94% and 83%/78%, respectively.
Conclusion
OPC patients with positive post-CRT PET exhibit poor survival. The prognostic accuracy of post-CRT PET is superior to that of CT. The results of post-CRT FDG-PET should be included in the management of the OPC patients.
Key words: FDG, PET, HNSCC, Oropharynx, Carcinoma, Prognosis, Chemoradiotherapy, Neck
Introduction
Squamous cell carcinoma of the oropharynx (OPC) is the most prevalent type of head and neck squamous cell carcinoma (HNSCC) and is increasing gradually, most likely due to the prolongation of the average life span and increases in cigarette smoking and alcohol consumption [1, 2]. The incidence in men is 2–5 times greater than that in women. The incidence and death of pharyngeal cancer is about 11,800 and 2,180 people in 2007, respectively, but this incidence varies markedly between countries [3].
The management of patients with OPC remains controversial. The predominant treatment modalities consist of surgery, radiation therapy (RT), and chemoradiotherapy (CRT), individually or in combination [4–10]. Although single-modality therapy with radiation or surgery can achieve similar loco-regional control for the early stage, patients with more advanced disease (stage III or IV) have been treated with surgery followed by RT or CRT. The survival figures for advanced-stage OPC have remained moderate despite the use of radical combined modality treatments. In recent meta-analysis for advanced OPC, tumor control and survival did not differ significantly between groups undergoing surgery with or without RT and RT with or without neck dissection (ND). However, patients who underwent surgery with or without RT exhibited a higher rate of severe or fatal complications [9]. Recently, the utility of concurrent CRT for OPC has been reported [10]. In terms of complications, RT with CRT could become a first-line treatment for OPC.
Outcome indicators in patients with HNSCC, including OPC, have traditionally been derived from clinical and pathologic features [11, 12]. The identification of additional prognostic factors for survival may allow the development of individualized strategies that lead to improved survival outcomes. Recent reports have described that pre-treatment positron emission tomography (PET) using 2-deoxy-[F-18]fluoro-d-glucose (FDG) for HNSCC including OPC predicted loco-regional control and survival outcome [13–17]. However, a limited number of reports have assessed the utility of post-CRT FDG-PET for HNSCC [18]. Herein, we report the importance of post-CRT FDG-PET as a novel prognostic indicator in patients with OPC.
Materials and Methods
Patients
Between March 2004 and April 2006, 24 consecutive patients with histologically proven OPC who were referred for PET evaluation of tumor metabolism were registered prospectively into a database. Six patients with systemic metastasis (Stage M1; two patients) at baseline investigation and who were treated with surgery (no consent of CRT; four patients) were excluded. Eighteen patients (14 men, four women; mean age ± SD, 61.7 ± 9.0 years) were ultimately enrolled in this study. A summary of the patient and tumor characteristics is summarized in Table 1. Written informed consent was obtained for all study patients.
Table 1.
Demographic and clinical characteristics of this study population (n = 18)
| Characteristics | No. of patients |
|---|---|
| Gender, male/female | 14/4 |
| Age, years (range) | 61.7 ± 9.0 (48–78) |
| Site of primary tumor | |
| Anterior wall | 3 |
| Lateral wall | 11 |
| Posterior wall | 3 |
| Superior wall | 1 |
| Histological grading | |
| G1/G2/G3 | 8/5/5 |
| TNM classification (AJCC 2002) | |
| T1/T2/T3/T4 | 2/4/9/3 |
| N0/N1/N2/N3 | 5/1/11/1 |
| M0/M1 | 18/0 |
| Stage I/II/III/IV | 0/1/5/12 |
G1/G2/G3 well/moderately/poorly differentiated, TNM tumor-node metastasis, AJCC American Joint Committee on Cancer
Concurrent CRT
External beam radiotherapy was administered to a total dose of 60 Gy in 30 fractions, delivered at five fractions per week for 6 weeks. Chemotherapy consisted of six cycles of cisplatin (20 mg/m2), docetaxel (10 mg/m2) and 5-fluorouracil (1,000 mg/m2). Chemotherapy was administered during week 1 of radiotherapy.
Assessment of the Tumor on Pre- and Post-CRT
All twenty patients underwent laryngoscopy, gastroscopy, chest X-ray, CT of the neck, and FDG-PET to assess the initial stage. Initial staging and treatment decisions did not take into consideration the results of the FDG-PET scan.
Six weeks after the completion of CRT, tumor restaging was performed with laryngoscopy, CT of the neck, and FDG-PET. Re-staging and additional treatment decisions did not take into account the assessment of the FDG-PET scan.
CT Scans of Neck
All pre- and post-CRT CT scans of the neck with contrast medium were acquired with helical scans in 5- to 10-mm slices. For the purpose of analysis, residual disease was classified in a dichotomous fashion as negative or positive, based on CT findings before and after CRT according to the Response Evaluation Criteria In Solid Tumors (RECIST) criteria [19]. A loco-regional post-CRT CT visual score was documented as either positive (either local or regional residual site) or negative (both local and regional non-residual site) by RECIST.
FDG-PET scans
A total of 36 FDG-PET scans were performed at pre- and post-CRT. Each patient fasted for at least 6 h before intravenous administration of approximately 370 MBq of FDG. The PET scans were performed 1 h after FDG injection using a dedicated scanner (HeadtomeV/ SET2400 W, Shimadzu, Kyoto, Japan), with 32 rings of bismuth germanate (BGO) detectors that simultaneously produces 63 slices 3.125 mm thick along a 20-cm longitudinal field. All emission data were corrected for tissue attenuation by measure of transmission scan with an external source of 68Ge–68Ga. The intrinsic resolution was 3.7 mm full width at half-maximum (FWHM), and the sensitivity of the device was 7.3 cps/Bq cm−3. Whole-body scans required four bed positions and were reconstructed using an iterative median root reconstruction algorithm.
High-resolution transaxial, coronal, sagittal, and maximal intensity projection images were displayed on a linear gray scale monitor. In pre-CRT PET assessment, the standardized uptake value (SUV) of the highest pixel within each local and regional site was calculated by dividing the decay-corrected maximal count of tumor area by the injected dose of FDG per unit of body weight. A loco-regional visual score (positive or negative) of FDG-PET was documented for the post-CRT PET assessment. The negative score indicated visually that there was no abnormal uptake at both the local and regional sites and a positive score indicated abnormal accumulation at either the local or regional site. All images were analyzed by two experienced nuclear medicine physicians and a consensus was reached away two readers in all patients.
Statistical Analysis
Kaplan–Meier analysis was used to evaluate survival. Cause-specific survival (CSS) and disease-free survival (DFS) were measured from the date of the pre-CRT PET scan to the date of death due to disease. The log-rank test was used to assess the correlation of the endpoints with the visual score of post-CRT PET and CT, and other clinical and pre-CRT PET variables (Gender, Age, T category, pre-CRT Local SUV, N category, pre-CRT Regional SUV, TNM stage, Histological grade). P-values less than 0.05 were considered to be statistically significant. All calculations were performed using Statcel software, version 2 (OMS Inc., Tokyo, Japan).
The positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy of conventional contrast-enhanced CT and FDG-PET at post-CRT for loco-regional recurrence including residual of disease were calculated to assess the predictive potential of these modalities.
Results
Response to CRT
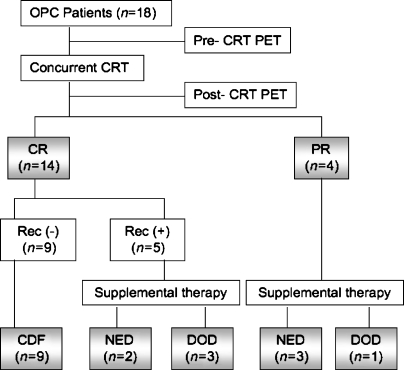
Figure 1 shows the response to CRT at last follow-up. In the eighteen OPC patients who were treated with concurrent CRT, 12 patients are still alive, four died as result of the disease and two died as a result of other primary cancers (esophageal and pancreatic carcinoma). The median follow-up for surviving patients was 24 months (range 9–37 months). Nine patients exhibited no recurrence after CRT. In nine additional patients, residual disease or recurrence was confirmed histologically and required salvage therapy. Two patients presented with local recurrence alone, five presented with regional disease, one patient presented with local and regional disease, and one patient presented with local recurrence and distant metastasis. At 2 years, local control was 78%, regional control was 72%, and CSS was 78%.
Fig. 1.
Flow chart showing the distribution of the patients' outcome. OPC squamous cell carcinoma of the oropharynx, CRT chemoradiotherapy, CR complete response, PR partial response, Rec recurrence, CDF continuous disease-free, NED no evidence of disease, DOD died of disease.
Patient Outcome
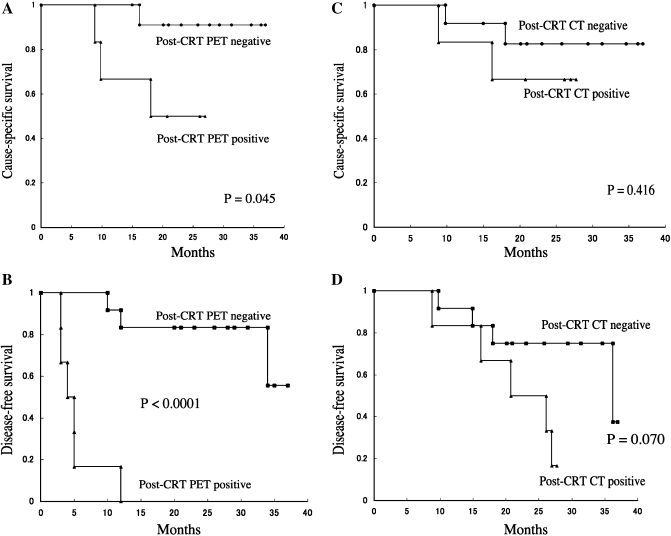
We evaluated the ability of pre- and post-CRT FDG-PET to predict long-term survival. In univariate analysis, patients with a positive visual score of post-CRT FDG-PET exhibited significantly lower 2-year CSS and DFS (50% vs. 91%, P = 0.045 and 0% vs. 83%, P < 0.0001), but patients with a positive RECIST score of the post-CRT CT scan did not exhibit a statistically lower 2-year CSS and DFS (67% vs. 83%, P = 0.416 and 50% vs. 75%, P = 0.070; Fig. 2). No other factors, clinical or pre-CRT PET variables differed regarding survival.
Fig. 2.
Cause-specific survival (CSS) and disease-free survival (DFS) according to the loco-regional score in post-CRT PET and CT scans. The patients with negative post-CRT PET had statistically superior CSS than patients with PET positive (a, P = 0.045). The positive post-CRT PET scan was a strong predictor of DFS (b, P < 0.0001). However, negative correlations were observed between post-CRT CT scan and survival (CSS and DFS) in OPC patients treated with concurrent CRT (c, P = 0.416; d, P = 0.070).
Residual and Recurrence Predictive Performance of Post-CRT FDG-PET and CT
The prognostic performance for post-CRT FDG-PET and CT is summarized in Table 2. Comparing post-CRT FDG-PET and CT findings for the assessment of recurrence at the primary site (local recurrence), both imaging modalities did not exhibit any false-positive results and three false-negative findings in four cases. However, the positive findings were found in different cases (Fig. 3). For those patients with lymph node metastases (regional recurrence), contrast-enhanced CT was false-positive in two cases, compared with no false-positive FDG-PET findings. CT was false-negative in two cases, compared with one case for FDG-PET (Fig. 4). The overall accuracy of post-CRT FDG-PET and CT in evaluating local recurrence was 83% and 83% respectively, and 94% versus 78% for regional recurrence.
Table 2.
Diagnostic accuracy of post-CRT assessment in local and regional recurrence of disease (n = 18)
| PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|
| Local recurrence | |||
| CT (+CE) | 100 (22–100) | 82 (78–82) | 83 (75–83) |
| FDG-PET | 100 (22–100) | 82 (78–82) | 83 (75–83) |
| Regional recurrence | |||
| CT (+CE) | 67 (36–86) | 83 (68–93) | 78 (57–91) |
| FDG-PET | 100 (67–100) | 92 (79–92) | 94 (76–94) |
Number in parentheses are 95% CI
PPV positive predictive value, NPV negative predictive value, CE contrast enhancement
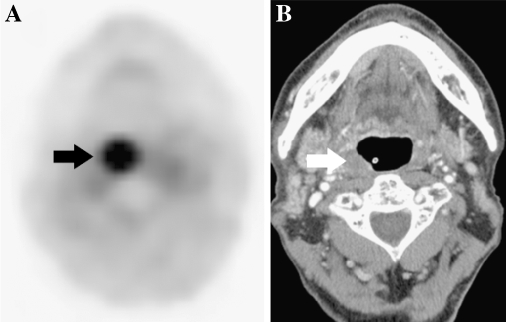
Fig. 3.
Discrepancy between post-CRT PET and CT findings in a 78-year-old male with OPC of the right lateral wall, tonsil, treated with concurrent CRT. Local recurrence was confirmed histologically about 6 months later. A contrast-enhanced CT scan resulted in false-negative findings. a In post-CRT PET, strong uptake tracer was seen the right lateral wall (arrow). b Post-CRT axial CT scan showed normal to slight hypertrophy of the tonsil without contrast enhancement (arrow), and histological specimen immediately after the end of CRT indicated no evidence of disease.
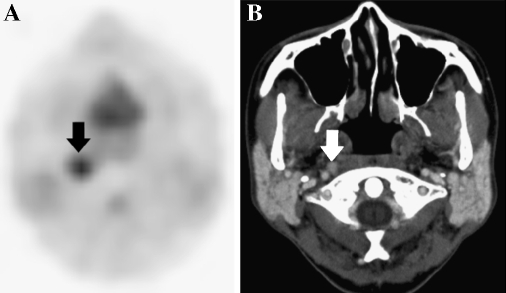
Fig. 4.
Discrepancy between PET and CT findings. A 48-year-old female with OPC of the superior wall relapsed in the right parapharyngeal site about 2 months later. A contrast-enhanced CT resulted in false-negative findings. a Post-CRT PET correctly identified the right parapharyngeal metastasis (arrow). b Post-CRT axial CT scan showed an 8 × 7 mm lymph node that was enhanced homogeneously (arrow) with no evidence of metastasis.
Discussion
The identification of prognostic factors for loco-regional control and survival may allow the development of individualized strategies to improve loco-regional control and survival. CT and MRI are major imaging tools in the management of patients with head and neck squamous cell carcinoma. Recently, several studies have suggested that pre-treatment FDG-PET could play such a role in HNSCC patients [13, 20]. The importance of the post-CRT metabolic response for loco-regional control and survival was reported in esophageal, rectal, and lung cancer patients [21–23]. Our multivariate analysis of the entire series of patients showed that, in the case of OPC, post-CRT FDG-PET was an independent prognostic factor for survival.
The current study confirmed that positive FDG-PET imaging after CRT identified a subset of OPC patients with poor prognosis. Elevated FDG uptake after CRT indicated the need to treat with salvage therapy for local, regional, or distant metastasis because of a strong correlation with DFS (P < 0.0001). The CSS indicated that even when OPC patients with positive FDG-PET after CRT are treated with additional salvage therapies, such as chemotherapy and surgery, their outcome is still poor. Post-CRT CT also tended to predict DFS and CSS, but this result was not statistically significant. Post-CRT strategies for OPC patients should be individualized not by conventional post-CRT CT scan but by post-CRT FDG-PET scan.
In the current study, there was no difference of CSS and DFS in the pre-CRT assessments. Many other studies have supported the importance of pre-treatment assessment for survival, including FDG-PET scan and TNM classification [11–13, 20]. Several reasons can be considered for the lack of correlation between the pre-CRT assessments and prognosis. Firstly, our study had been biased against the clinical staging because of a clinical feature of OPC that is difficult in early detection. Secondly, the numbers of our prospective samples were too small to assess the correlation between pre-treatment indicators and survival. A larger scale trial may be able to further investigate these correlations.
The prognostic accuracy of regional recurrence for post-CRT FDG-PET was superior to that of CT scan (94% vs. 78%). The clinical utility of post-treatment FDG-PET for regional disease has been reported by Porceddu et al. [24]. They indicated that HNSCC patients with negative PET scans, approximately 12 weeks after CRT, did not require surgery and could be observed safely. Our results for OPC patients are compatible with their findings. Early surgical treatment, such as neck dissection, should not be considered in OPC patients with negative FDG-PET after CRT.
In conclusion, the predictive value of post-CRT FDG-PET is superior to that of conventional CT scan in OPC patients. The OPC patients with positive FDG-PET after CRT exhibited poor survival. In contrast, negative PET studies after CRT indicated good prognoses. The results of post-CRT FDG-PET should be included in the management of the OPC patients.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman DV, Crossett LA, Ries LAG, Goldberg HI, Lockwood SA, Swango PA. Cancers of the oral cavity and pharynx: a statistics review monograph 1973–1987. Public Health Service, Atlanta, GA: USDHHS; 1991. [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–3977. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 5.Schuller DE, Ozer E, Agrawal A, Grecula JC, Rhoades CA, Young DC. Multimodal intensification regimens for advanced, resectable, previously untreated squamous cell cancer of the oral cavity, oropharynx, or hypopharynx: a 12-year experience. Arch Otolaryngol Head Neck Surg. 2007;133:320–326. doi: 10.1001/archotol.133.4.320. [DOI] [PubMed] [Google Scholar]
- 6.Schuller DE, Grecula JC, Agrawal A, et al. Multimodal intensification therapy for previously untreated advanced resectable squamous cell carcinoma of the oral cavity, oropharynx, or hypopharynx. Cancer. 2002;94:3169–3178. doi: 10.1002/cncr.10571. [DOI] [PubMed] [Google Scholar]
- 7.Licitra L, Bernier J, Grandi C, Merlano M, Bruzzi P, Lefebvre JL. Cancer of the oropharynx. Crit Rev Oncol Hematol. 2002;41:107–122. doi: 10.1016/S1040-8428(01)00129-9. [DOI] [PubMed] [Google Scholar]
- 8.Klug C, Wutzl A, Kermer C, et al. Preoperative radiochemotherapy and radical resection for stages II–IV oral and oropharyngeal cancer: outcome of 222 patients. Int J Oral Maxillofac Surg. 2005;34:143–148. doi: 10.1016/j.ijom.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967–2980. doi: 10.1002/cncr.10567. [DOI] [PubMed] [Google Scholar]
- 10.Kokubo M, Nagata Y, Nishimura Y, et al. Concurrent chemoradiotherapy for oropharyngeal carcinoma. Am J Clin Oncol. 2001;24:71–76. doi: 10.1097/00000421-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 11.de Cassia Braga Ribeiro K, Kowalski LP, Latorre Mdo R. Perioperative complications, comorbidities, and survival in oral or oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2003;129:219–228. doi: 10.1001/archotol.129.7.739. [DOI] [PubMed] [Google Scholar]
- 12.Pugliano FA, Piccirillo JF, Zequeira MR, et al. Clinical-severity staging system for oropharyngeal cancer: five-year survival rates. Arch Otolaryngol Head Neck Surg. 1997;123:1118–1124. doi: 10.1001/archotol.1997.01900100094013. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Roh JL, Kim MR, et al. Use of 18F-FDG PET for primary treatment strategy in patients with squamous cell carcinoma of the oropharynx. J Nucl Med. 2007;48:752–757. doi: 10.2967/jnumed.107.039610. [DOI] [PubMed] [Google Scholar]
- 14.Kim MR, Roh JL, Kim JS, et al. Utility of 18F-fluorodeoxyglucose positron emission tomography in the preoperative staging of squamous cell carcinoma of the oropharynx. Eur J Surg Oncol. 2007;33:633–638. doi: 10.1016/j.ejso.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DL, Rajendran J, Yueh B, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–1367. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- 16.Sanghera B, Wong WL, Lodge MA, et al. Potential novel application of dual time point SUV measurements as a predictor of survival in head and neck cancer. Nucl Med Commun. 2005;26:861–867. doi: 10.1097/00006231-200510000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Dobert N, Kovacs AF, Menzel C, et al. The prognostic value of FDG PET in head and neck cancer. Correlation with histopathology. Q J Nucl Med Mol Imaging. 2005;49:253–257. [PubMed] [Google Scholar]
- 18.Nayak JV, Walvekar RR, Andrade RS et al Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. in press [DOI] [PubMed]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Roh JL, Pae KH, Choi SH, et al. 2-[18F]-Fluoro-2-deoxy-d-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007;33:790–795. doi: 10.1016/j.ejso.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-d-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–1785. doi: 10.1002/cncr.20585. [DOI] [PubMed] [Google Scholar]
- 22.Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47:14–22. [PubMed] [Google Scholar]
- 23.Eschmann SM, Friedel G, Paulsen F, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:463–471. doi: 10.1007/s00259-006-0273-5. [DOI] [PubMed] [Google Scholar]
- 24.Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27:175–181. doi: 10.1002/hed.20130. [DOI] [PubMed] [Google Scholar]