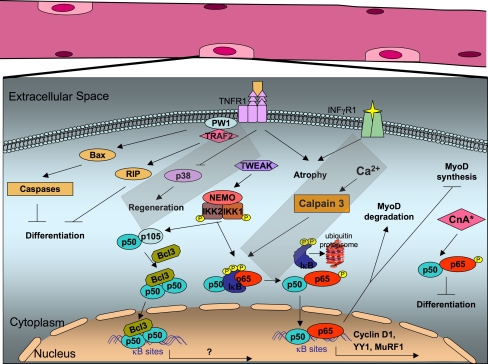
Fig. 2.
NF-κB pathway in skeletal muscles. NF-κB binds on κB sites of the cyclin D1 promoter and regulates its transcription. Moreover, the p65/p50 heterodimer complex binds to the transcriptional repressor YY1, resulting in inhibition of skeletal myogenesis. TNF-α and TWEAK activation regulates MyoD1 expression through a p65/p50 complex. In response to TNF signaling, PW1 associates with TRAF2, induces Bax translocation in mitochondria, and through activation of caspases, leads to inhibition of muscle differentiation. TNF-α signaling is important for the activation of satellite cells during muscle regeneration, through the MAP kinase p38. Synergistic effects of TNF and INF-γ result in muscle atrophy. Stable expression of constitutively active CnA in C2C12 cells induces NF-κB activation in a TNF-α-independent mechanism. Intracellular calcium in muscle cells activates calpain 3, which induces IκBα degradation, leading to NF-κB activation and translocation into the nucleus, where it regulates expression of survival genes. Upon denervation-induced atrophy, NF-κB binds on the promoter of MuRF1. Upon unloaded-induced atrophy, complexes comprising of p50 and Bcl-3 subunits are activated and translocate into the nucleus to regulate transcription of target genes. MuRF1 Murine ring finger-1, YY1 YinYang1, PW1/Peg3 paternally expressed 3, TWEAK TNF weak inducer of apoptosis, CnA activated form of calcineurin A, Bax Bcl-2-associated X protein, TRAF2 TNF-receptor-associated factor 2, RIP receptor-interacting protein, TNFR1 tumor necrosis factor receptor 1, INF-γR1 interferon-γ receptor 1, IKK IκB kinase, NEMO NF-κB essential modulator