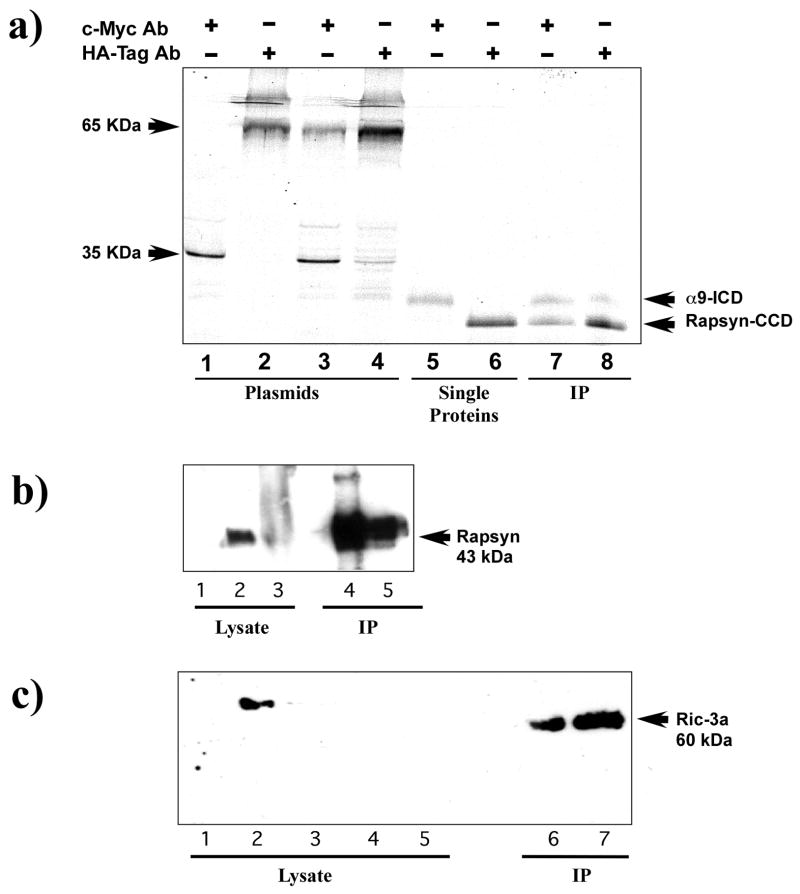
Figure 2.
Immunopreciptitation of nAChR α9 subunit interactions with rapsyn and RIC-3. (a) An autoradiograph of in vitro expression of S35 methionine labeled proteins of the intracellular domain of the nAChR α9 subunit (α9-ICD) and the coiled-coil domain of rapsyn (rapsyn-CCD). Lanes 1–4 contain internal positive controls of murine p53 (c-Myc epitope) and SV40 large T-antigen (HA epitope) that were used in the for the yeast two-hybrid screen. They show that precipitation of either the murine p53 or the large T-antigen results in a single band (lanes 1 and 2) while immunoprecipitation of murine p53 mixed with large T-antigen results in two bands (lanes 3 and 4). Similarly, lanes 5 and 6 show that the nAChR α9-ICD labeled with the c-Myc epitope is recognized by the c-Myc antibody and rapsyn-CCD labeled with the HA epipitope is recognized by the HA antibody. Lanes 7 and 8 are experimental co-immunoprecipitation lanes. The c-Myc antibody (lane 7) precipitated labeling of both α9-ICD and rapsyn-CCD. Similarly, in lane 8 the HA antibody precipitated labeling of both α9-ICD and rapsyn-CCD fragments. The authenticity of the rapsyn-HA and α9-myc fragments were confirmed by Western blot. (b) Immunoblot analysis of the expression of full-length nAChRα9 subunit and rapsyn in HEK293T cells and mouse cochlea homogenates. nAChR α9 subunit was immunoprecipated with α9 antibody and immunoblots then probed with rapsyn antibody. A specific band corresponding to the 43-kDa mouse rapsyn was detected in α9 and rapsyn co-transfected HEK293T cells (lane 4) and observed in lysate obtained from mouse cochleae (lane 5). Positive controls from HEK293T cells transfected with rapsyn alone (lane 1), mouse muscle (lane 2) and cochlea (lane 3) were used. (c) Immunoprecipitation (IP) of nAChR α9 subunit followed by immunoblot analysis with RIC-3a antibody. As shown: lane 1 (HEK293T cells untransfected), lane 2 (HEK293T cells transfected with RIC-3 cDNA), lane 3 (QT-6 cells untransfected), lanes 4 and 5 (mouse and rat cochlea lysates, respectively). HEK392 cells co-transfected with AChRα9 subunit and RIC-3 cDNAs and then immunoprecipitated with AChRα9 antibody (lane 7), show a band of ~ 60 kDa, consistent with the RIC-3 expected molecular size. A similar band was also observed from rat cochlea that was immunoprecipitated AChRα9 antibody (lane 6).