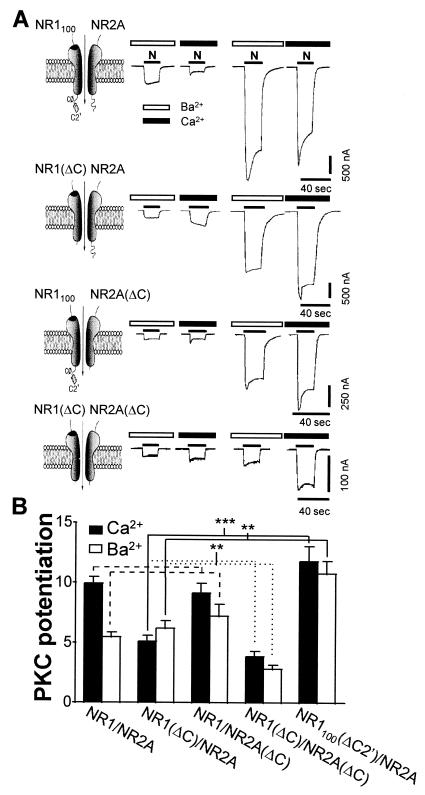
Figure 4.
The C-terminal tails of NR1 and NR2 are not required for PKC potentiation. (A) Responses of NR1100/NR2A, NR1(ΔC)/NR2A, NR1100/NR2A(ΔC), and NR1(ΔC)/NR2A(ΔC) receptors in Ba2+ and Ca2+ Ringer's solution before and after TPA treatment. Responses of NR1(ΔC)/NR2A receptors were larger in Ca2+ than in Ba2+ Ringer's solution and increased during agonist application. Basal currents of NR1/NR2A(ΔC)and NR1(ΔC)/NR2A(ΔC) receptors were small relative to those of wild-type receptors. (B) Mean PKC potentiation of mutant and wild-type receptors illustrated in A and of NR1100(ΔC2′)/NR2A receptors. NR1(ΔC)/NR2A receptors showed significantly less potentiation than did wild-type NR1100/NR2A receptors (which contain the C0 region and C2′ splice cassette) in Ca2+ Ringer's solution (P < 0.001) but did not differ significantly in Ba2+ Ringer's solution. The apparent reduction in the degree of potentiation could be accounted for by the larger basal current of the truncation mutant. NR1(ΔC)/NR2A receptors showed less potentiation than did NR1100(ΔC2′)/NR2A receptors (which contain the C0 region) in Ca2+ (P < 0.001) and Ba2+ Ringer's solution (P < 0.01). Potentiation of NR1100/NR2A(ΔC) receptors did not differ significantly from that of wild-type receptors. The double-truncation mutant NR1(ΔC)/NR2A(ΔC) showed significantly less PKC potentiation that did either single truncation mutant [P < 0.01 vs. NR1(ΔC)/NR2A and vs. NR1100/NR2A(ΔC)]. PKC potentiated responses of NR1100/NR2A, NR1(ΔC)/NR2A, NR1100/NR2A(ΔC), NR1(ΔC)/NR2A(ΔC), and NR1(ΔC2′)/NR2A receptors to 9.9 ± 0.6 (n = 24), 5.1 ± 0.5 (n = 16), 9.1 ± 0.9 (n = 7), 3.9 ± 0.5 (n = 6), and 11.8 ± 0.1.3 (n = 10) times control in Ca2+ and to 5.5 ± 0.5 (n = 19), 6.2 ± 0.7 (n = 11), 7.2 ± 1.1 (n = 7), 2.8 ± 0.4 (n = 6), and 10.8 ± 1.1 (n = 10) times control in Ba2+ Ringer's solution. ***, P < 0.001; **, P < 0.01; *, P < 0.05.