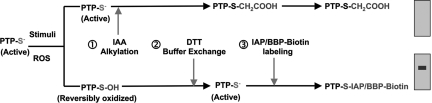
Fig. 1.
Schematic outline of the cysteinyl-labeling assay. An active PTP is indicated with the active-site cysteinyl residue as a thiolate anion, representing the cell at a resting state. (1) After a physiological stimulus, the cells were lysed in a degassed buffer at pH 5.5 containing IAA. The low-pKa Cys residue at the active site of those PTPs that remained in a reduced state are alkylated, terminally inactivating this pool of PTPs. Conversely, the Cys residues that were oxidized by second-messenger ROS molecules were protected from irreversible alkylation. (2) IAA was then removed from the lysate by buffer exchange using size-exclusion spin columns, and the oxidized Cys residues were reduced back to the thiolate ion with DTT. (3) The oxidized PTPs were maintained in pH 5.5 buffers and incubated with either of two biotinylated, active-site-directed compounds, a sulfhydryl-reactive IAP probe or a BBP activity-based probe. Purification by streptavidin pull-down and immunoblotting permits identification of ROS-targeted PTPs.