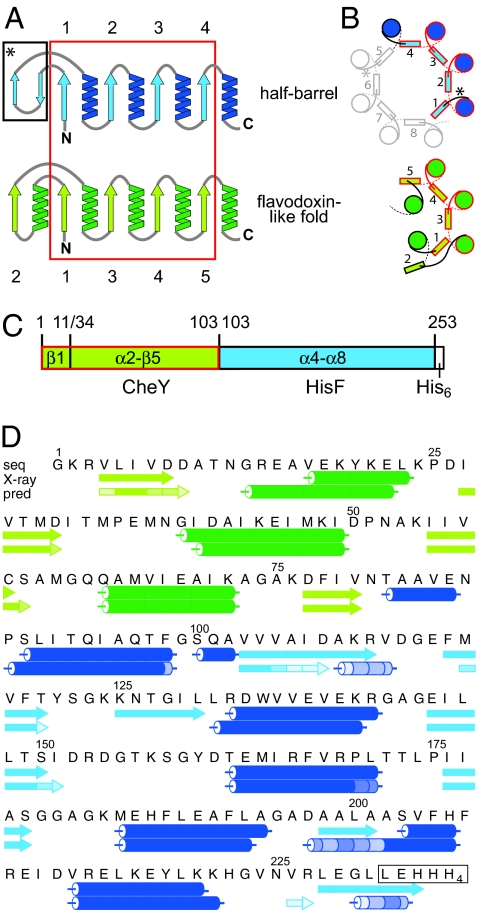
Fig. 1.
Structural comparison of half-barrels with proteins of the flavodoxin-like fold and construction of the CheYHisF chimera. (A) Topological diagram of the half-barrels [(βα)1–4, blue] and the flavodoxin-like (βα)5-fold (green) (13). The structurally superimposable parts are encircled in red. β-strands are numbered. (B) Comparison of the spatial arrangement of the main secondary structural elements in (βα)8-barrel and flavodoxin-like proteins (top view). α-Helices are depicted as circles, and β-strands are shown as rectangles. Colors and numbering are as in A. The extra two-stranded β-sheets in the (βα)8-barrel are omitted for clarity; their positions are indicated by asterisks. (C) Construction of the CheYHisF chimera. The fragments originating from CheY (residue 1–11 including β1 and 34–103 including α2-β5) are depicted in green, and the fragment originating from HisF (residue 103 to 253 including α4-β8) is depicted in blue. (D) Amino acid sequence and secondary structure of the CheYHisF construct with the attached tag (boxed). Underneath the secondary structure elements are shown as observed in the crystal structure and as predicted by the program psipred (19). The lighter the shade of color in each element the smaller is the confidence of the prediction.