Figure 1.
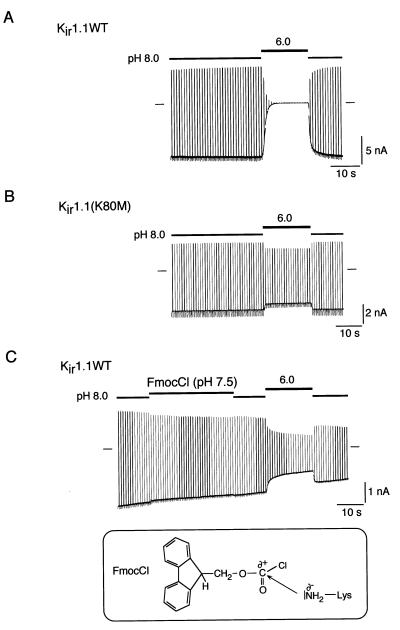
pH gating of Kir1.1 channels is driven by protonation of Lys-80 in the neutral pH range. (A) pH gating of Kir1.1 channels measured in a giant inside-out patch from Xenopus oocytes on switching pHi from 8.0 to 6.0 (inactivation) and from 6.0 to 8.0 (recovery from inactivation). Currents were recorded at a membrane potential of −80 mV, which was stepped up to 50 mV for 50 ms every 0.9 s. Solution application and changes in pHi are illustrated by horizontal bars, time and current scales as indicated. (B) Same experiment as in A but with the pH-insensitive mutant Kir1.1(K80M) in which Lys-80 was replaced by methionine. (C) (Upper) pH gating is abolished in Kir1.1 channels by application of FmocCl at pHi 7.5 before acidification. Changes in pHi and application of FmocCl as indicated by horizontal bars. (Lower) Structure of FmocCl and its chemical reaction (nucleophilic substitution) with the amino group of a lysine residue.