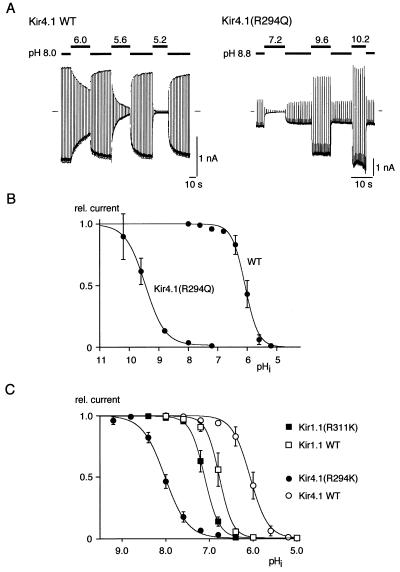
Figure 3.
Sensitivity of pH gating to side-chain variations at the C-terminal residue in Kir4.1 and Kir1.1 channels. (A and B) Shift in pH gating induced by neutralization of the C-terminal residue in Kir4.1 channels. (A) Recordings from Kir4.1 wild-type and Kir4.1(R294Q) channels at the pHis indicated; experimental protocol as in Fig. 1. (B) Current-pHi relation of Kir4.1(R294Q) and Kir4.1 wild type. Note the shift in pH0.5 observed for the mutant channels with respect to wild type [pH0.5 and Hill coefficient were 9.5 and 1.4 for Kir4.1(R294Q) and 6.1 and 2.1 for Kir4.1 wild type]. (C) Effect of an Arg-Lys exchange at the C-terminal site in Kir1.1 and Kir4.1 channels on pH gating. Current-pHi relation obtained from Kir1.1 wild type and Kir1.1(R311K) (squares) and from Kir4.1 wild type and Kir4.1(R294K) (circles). Lines represent fit of a Boltzmann function to the data (mean ± SD of eight and seven experiments for R311K and R294K, respectively). pH0.5 and Hill coefficient, respectively, were 7.1 and 2.7 for Kir1.1(R311K) and 8.0 and 1.7 for Kir4.1(R294K).