Abstract
Experiments were done in urethane-anesthetized, barodenervated, male Wistar rats. Chemical stimulation of the hypothalamic paraventricular nucleus (PVN) by unilateral microinjections of N-methyl-D-aspartic acid (NMDA) elicited increases in mean arterial pressure (MAP) and greater splanchnic nerve activity (GSNA). The increases in the MAP and GSNA induced by chemical stimulation of the PVN were significantly exaggerated by bilateral microinjections of D-AP7 and NBQX (ionotropic glutamate receptor antagonists) into the medial subnucleus of the nucleus tractus solitarius (mNTS). These results were confirmed by single unit recordings; i.e., excitation of mNTS barosensitive neurons caused by chemical stimulation of the ipsilateral PVN was blocked by application of D-AP7 and NBQX to these neurons. Bilateral microinjections of D-AP7 and NBQX into the mNTS elicited pressor responses which were significantly attenuated by inhibition of PVN neurons by bilateral microinjections of muscimol. Unilateral microinjections of fluorogold into the mNTS resulted in bilateral retrograde labeling of the PVN neurons. Unilateral microinjections of biotinylated dextran amine into the PVN resulted in anterograde labeling of axons and terminals in the mNTS bilaterally and the labeled terminals exhibited vesicular glutamate transporter-2 immunoreactivity. These results indicated that; 1) a tonically active glutamatergic bilateral projection from the PVN to the mNTS exists, 2) bilateral blockade of ionotropic glutamate receptors in the mNTS exaggerates the increases in MAP and GSNA to the chemical stimulation of the PVN, and 3) this projection may serve as a restraint mechanism for excitatory cardiovascular effects of PVN stimulation.
Keywords: blood pressure, barodenervation, ionotropic glutamate receptors, microinjections, sympathetic nerve activity, VGLUT2
INTRODUCTION
Although there is a general consensus that the rostral ventrolateral medullary pressor area (RVLM) is of critical importance in the regulation of cardiovascular function (Guyenet, 2006; Sapru, 2002), emerging evidence indicates that hypothalamic paraventricular nucleus (PVN) also plays a significant role in controlling cardiovascular function (Badoer, 2001; Coote, 2004; Li et al., 2006; Shen et al., 1992). The nucleus tractus solitarius (NTS) is another area that is important in cardiovascular regulation (Sapru, 2004). It is well established that the peripheral baroreceptor, chemoreceptor and cardiopulmonary afferents make their first synapse in the NTS. The secondary NTS neurons send an excitatory projection to the caudal ventrolateral medullary depressor area (CVLM), which, in turn, sends an inhibitory GABAergic projection to the RVLM (Guyenet, 2006; Sapru, 2002). The NTS is known to receive projections from the anterior, medial and lateral parvocellular cells of the PVN while the dorsal parvocellular cells project primarily to the spinal cord (Hardy, 2001; Luiten et al., 1985; Palkovits, 1999; Swanson and Sawchenko, 1983).
The descending projections from the PVN to the NTS are believed to be predominantly peptidergic (Palkovits, 1999; Sofroniew and Schrell, 1981) and oxytocin and vasopressin have been implicated as neurotransmitters or neuromodulators in these projections (Buijs, 1978). Specific activation of vasopressinergic and/or oxytocinergic supra-medullary pathways has been implicated in adjustment of heart rate (HR) and cardiac output during rest and exercise (Michelini, 2007). For example, treadmill running elicits an increase in the vasopressin content in the dorsal brain stem, resulting in the activation of V1 receptors and causing significant improvement in exercise-induced tachycardia (Dufloth et al., 1997). Oxytocinergic input to the NTS has been reported to be tonic and activation of this pathway during exercise improves reflex bradycardia by facilitating vagal influence on the heart (Braga et al. 2000; Higa et al., 2002; Michelini, 2007). The peptidergic projections from the PVN to the NTS may also mediate functions other than cardiovascular regulation. Indeed, it has been reported that the peptidergic projections from the PVN to the NTS may be involved in facilitating the effects of visceral afferent input from the abdominal vagus to the NTS (Banks and Harris, 1987; Kannan and Yamashita, 1985; Rogers and Hermann, 1985).
Since it is firmly established that glutamate is involved in the reflex regulation of cardiovascular function in the NTS (Gordon and Sved, 2002; Guyenet, 2006; Sapru, 2002, 2004; Talman et al., 1984), it was hypothesized that, in addition to vasopressin and oxytocin, this neurotransmitter may also be involved in the hypothalamic modulation of cardiovascular function in the NTS. In the present paper, we report the presence of a glutamatergic projection from the PVN to the NTS and characterization of its cardiovascular function.
EXPERIMENTAL PROCEDURES
General procedures
Experiments were done in adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 300–380 g (n = 73). All animals were housed under controlled conditions with a 12:12-h light-dark cycle. Food and water were available to the animals ad libitum. The experiments were performed according to the NIH guide for “The Care and Use of Laboratory Animals, 7th Edition, 1996” and with the approval of the Institutional Animal Care and Use Committee of this university.
The general procedures have been described in detail elsewhere (Kawabe et al., 2006). Briefly, the rats were anesthetized with inhalation of isoflurane (2–3% in 100% oxygen), one of the veins was cannulated and urethane (1.2–1.4 g/kg) was injected intravenously in 8–9 aliquots at 2-min intervals (total volume of the anesthetic solution was 0.4–0.45 ml injected over a period of about 16–18 min). Isoflurane inhalation was terminated as soon as urethane administration was completed. Absence of a BP response and/or withdrawal of the limb in response to pinching of a hind paw indicated that the rats were properly anesthetized. One of the arteries was cannulated for monitoring BP. HR was monitored by a tachograph that was triggered by the BP waves. Mean arterial pressure (MAP) was derived electronically. The tidal volume and frequency were adjusted on the ventilator to maintain the end tidal CO2 at 3.5–4.5%. Rectal temperature was maintained at 37 ± 0.5°C. All the tracings were recorded on a polygraph (model 7D, Grass Instruments, West Warwick, RI, USA).
Barodenervation
Barodenervated rats were used in most of the physiological experiments unless indicated otherwise. Barodenervation was accomplished by bilateral section of the carotid sinus, aortic depressor and recurrent laryngeal nerves. Subsequent bolus injection of phenylephrine (4 µg/kg, i.v.) failed to elicit reflex bradycardia indicating that the barodenervation was complete.
Microinjection technique
The details of this technique are described elsewhere (Kawabe et al., 2006). Briefly, the rats were placed in a prone position in a stereotaxic instrument with bite bar 11 mm below the interaural line. The bregma was visually identified and a small hole was drilled in the parietal bone. Multi-barreled glass-micropipettes (tip size 20–40 µm) were used for microinjections. The coordinates for microinjections into the PVN were: 1.5–2.0 mm caudal to the bregma, 0.4–0.5 mm lateral to the midline, and 7.7–8.0 mm deep from the dura. For microinjections into the medial subnucleus of the NTS (mNTS) in the same rat, the coordinates for microinjections into the mNTS were: 0.5–0.6 mm rostral to the calamus scriptorius, 0.5–0.6 mm lateral to the midline and 0.5–0.6 mm ventral to the dorsal surface of the medulla. The PVN was identified by unilateral microinjections of N-methyl-D-aspartic-acid (NMDA; 10 mM) and the mNTS was identified by microinjecting L-glutamate (L-Glu; 5 mM). The volume of NMDA or (±)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) microinjected into the PVN unilaterally was 50 nl unless indicated otherwise. The duration of microinjection was 10 sec. Controls for microinjections consisted of artificial cerebrospinal fluid (aCSF, pH 7.4).
Greater splanchnic nerve recording
The details of recording from the greater splanchnic nerve are mentioned elsewhere (Kawabe et al., 2006). Briefly, the greater splanchnic nerve (GSN) was sectioned at its junction with the celiac ganglion, a small segment was desheathed and its activity was recorded using a bipolar hook electrode made of silver wire. The whole GSN activity was amplified (X10,000–20,000) and filtered (100–5000 Hz), digitized and stored on a computer hard drive. The digitized signals were full-wave rectified and integrated over consecutive 1 sec intervals using Spike 2 program (CED). At the end of the experiment, the nerve was sectioned centrally and the remaining activity was considered to be the noise level which was subtracted from the GSNA amplitude.
Extracellular neuronal recording
Five-barreled glass micropipettes (Medical Systems, Greenvale, NY, USA) were used. The tip size was adjusted so that the resistance of the barrels was 4–8 mega Ohm. In this configuration, the recording barrel is in the center of the micropipette tip (approximately 5–10 µm) and the ejection barrels surround it. The central barrel used for recording was filled with 4M NaCl and other barrels contained aCSF, L-Glu, NBQX and D-AP7. The micropipette was inserted into the mNTS using the coordinates mentioned earlier. The spontaneous NTS neuronal extracellular activity was filtered (100–10,000 Hz) and amplified (X10,000–20,000) (using model DAM-80 amplifier, World Precision Instruments, Sarasota, FL, USA). The amplified signals were fed into a window discriminator (Frederick Haer, Brunswick, ME, USA) to generate TTL pulses which were fed into a rate counter (model RIC-830, Charles Ward, Ardmore, PA, USA). In the rate mode at 1 sec setting, the rate counter generated an analog signal indicating the frequency of firing as spikes/sec. The signal was digitized (using 1401 A/D converter, CED, Cambridge, UK) and stored on a computer hard drive. Analysis of these activities is described under statistical calculations. Ejection of the contents in different barrels on neurons was accomplished by application of pressure pulses (15 msec duration, 20 psig) and the volume of ejected solution (4 nl) was visually confirmed under a modified binocular horizontal microscope. Controls consisted of 4 nl of aCSF (pH 7.4). The involvement of recorded mNTS neurons in cardiovascular regulation was ascertained by their responses to the electrical stimulation of the ipsilateral aortic nerve (AN).
Aortic nerve stimulation
The procedure for AN stimulation is described elsewhere (Sapru et al., 1981). Briefly, either the left or right AN was sectioned at a level close to the chest cavity and the caudal end of the nerve was stimulated electrically using a bipolar silver electrode connected to a stimulator (model S88, Grass Instruments) via an isolation unit (model SIU5A, Grass Instruments) and constant current unit (model CCU-1, Grass Instruments). The parameters of the AN stimulation were 50–100 µA, 0.1 msec and 1 Hz. Using these parameters, two shocks were delivered to the nerve within an interval of 2 sec.
Retrograde tracing of PVN projections
PVN projections from the PVN to the mNTS were traced retrogradely by unilateral microinjections of fluorogold in the mNTS. The surgery for the tracing studies was done under aseptic conditions. The rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.), fixed in a prone position in a stereotaxic instrument and fluorogold (4–6%, 15 nl, Fluorochrome Inc., Denver, CO, USA) was microinjected into the mNTS on one side using the coordinates mentioned earlier. Absorbable gelatin sponge (Surgifoam, Ethicon Inc., Somerville, NJ, USA) was placed on the medullary surface, and the muscles and skin over the wound were sutured. The rats were kept alive for 5 days. An antibiotic (Cefazolin, 30 mg/kg) and an analgesic (Buprenorphine, 0.05 mg/kg) were administered subcutaneously twice a day for 3 days. The animals were then deeply anesthetized with pentobarbital (80 mg/kg, i.p.), perfused and fixed with transcardiac administration of 4% paraformaldehyde solution, and the brains were removed and fixed in 4% paraformaldehyde for 72 hrs. On completion of the fixation procedure, the left side of the brain surface was marked by a shallow cut and serial sections of the medulla and the hypothalamic area were cut (40 µm) in a vibratome (1000 Plus Sectioning System, The Vibratome Company, St. Louis, MO, USA), mounted on slides using Citifluor Mountant Media (Ted Pella Inc., Redding, CA, USA) and the sections were coverslipped. The microinjection site of fluorogold and the retrogradely-labeled cells were visualized under a microscope (model AX70, Olympus Provis, Middlebush, NJ, USA) equipped with a filter (Amax = 360 nm, Emax = 515 nm). The sections were photographed (using Neurolucida software, version 7.5, MicroBrightField Inc., Williston, VT, USA) and compared with a standard atlas (Paxinos and Watson, 1986).
Anterograde tracing of PVN projections and immunohistochemical detection of vesicular glutamate transporter-2
The general procedure for these experiments was identical to that described for retrograde tracing. In pentobarbital-anesthetized rats (n = 4), a double barreled micropipette was placed in the PVN. NMDA (10 mM) was microinjected (10 nl) through one barrel which was immediately followed by unilateral microinjection (10 nl) of biotinylated dextran amine (BDA-10,000, 10%, Invitrogen-Molecular Probes, Eugene, OR, USA). This procedure was repeated 10 times at 5 min intervals. The rats were kept alive for 14 days. The animals were then anesthetized, perfused and fixed as described earlier, and the sections were cut (30 µm) in a cryostat (CM1900, Leica Microsystems Inc., Bannockburn, IL, USA). The sections containing the NTS were blocked with 10% horse serum in Tris buffered saline (TBS), and incubated with 0.1% Triton X-100 for 60 min. The sections were then incubated with guinea pig anti-vesicular glutamate transporter-2 (VGLUT2) antibody (1:2,500, Chemicon International Inc., Temecula, CA, USA) for 12 hours at 4°C. After rinsing with TBS, the sections were incubated with Cy3-goat anti-guinea pig IgG (1:250, Amax = 550 nm, Emax = 570 nm, Jackson Immuno-Research Laboratories, Inc., West Grove, PA, USA) and streptavidin Alexa Fluor 488 conjugate (1:250, Amax = 495 nm, Emax = 519 nm, Invitrogen-Molecular probes) for 2 hours. After rinsing with TBS, the sections were mounted on subbed slides, covered with Citifluor and coverslipped. The images of the sections were captured, 2 µm apart, by laser scanning confocal microscopy (LSM510, Carl-Zeiss Inc.). For visualizing the BDA-microinjection site, the sections containing the PVN were treated with AB solution (ABC kit, Vector Laboratories Inc., Burlingame, CA, USA) for 2 hours. After rinsing, the sections were reacted with DAB Substrate Kit for Peroxidase (Vector Laboratories) for 10 min, rinsed, mounted on subbed slides, dehydrated through a series of graded alcohols, cleared in xylene, stained with cresyl violet and covered with Permount (Fisher Scientific, Suwanee, GA, USA) followed by a coverslip.
Histological identification of microinjection sites
Typical sites of microinjections in the PVN and mNTS were marked by microinjections of diluted India ink. The animals were perfused and fixed with 4% paraformaldehyde and serial sections of the medulla were cut (40 µm) in a vibratome, mounted on slides, dehydrated, cleared and stained with cresyl violet. The microinjection sites were identified under a microscope. The sections were photographed and compared with a standard atlas (Paxinos and Watson, 1986).
Drugs and chemicals
The following drugs and chemicals were used. N-methyl-D-aspartic acid (NMDA), D(−)-2-amino-7-phosphono-heptanoic acid (D-AP7; NMDA receptor antagonist), L-glutamate monosodium (L-Glu), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]quinoxaline-7-sulfonamide disodium (NBQX disodium salt; non-NMDA receptor antagonist), muscimol hydrobromide (GABAA receptor agonist), (±)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), L-phenylephrine hydrochloride, S(−)-atenolol, isoflurane, urethane, pentobarbital sodium, cefazolin and buprenorphine hydrochloride. All of the solutions for the microinjections were freshly prepared in aCSF. The composition of aCSF (pH 7.4) was as follows; the concentration (mM) of each constituent is mentioned in parenthesis: NaCl (128), KCl (3), CaCl2 (1.2), MgCl2 (0.8), dextrose (3.4) and HEPES (5). Where applicable, the concentration of drugs refers to their salts. The vendors for different drugs and chemicals were as follows: Isoflurane (Baxter Pharmaceutical Products, Deerfield, IL, USA), pentobarbital (Ovation Pharmaceuticals Inc., Deerfield, IL, USA), cefazolin (West-ward Pharmaceutical Corp., Eatontown, NJ, USA), buprenorphine (Hospira Inc., Lake Forest, IL, USA). All other drugs and chemicals were obtained from Sigma Chemicals (St. Louis, MO, USA).
Statistical analyses
The means and standard error of the means (SEM) were calculated for maximum change in MAP and HR in response to microinjections of different drugs. One-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparison test was used for concentration-response study, and determination of tachyphylaxis. Student’s paired t-test was used for comparison of the following responses: increases in MAP, HR induced by the microinjection of NMDA into the PVN before and after intravenous injections of atenolol or the microinjections of NBQX and D-AP7 into the mNTS. The same test was used for comparison of increases in MAP induced by microinjections of NBQX and D-AP7 into the mNTS before and after microinjections of muscimol into the PVN. For analyses of the GSNA, the integrated signals obtained just before the microinjections of NMDA into the PVN were averaged over a period of 60 sec. When the responses to these treatments were maximal, the integrated signals were averaged over a period of 60–90 sec. The percentage changes in sympathetic activities elicited by these treatments were calculated and compared by using Student’s paired t-test. For the analyses of the extracellular neuronal recordings, the basal firing rate and maximum firing rates in response to different manipulations (e.g., PVN stimulation, application of different drugs) were averaged over a 1 sec period and the comparisons of the firing frequencies of spikes before and after these manipulations were made by Student’s paired t-test. In all cases, the differences were considered significant at P < 0.05.
RESULTS
Concentration-response of NMDA in the PVN
Unilateral microinjections of NMDA into the PVN elicited increases in MAP and HR (Table. 1A). Maximal pressor and tachycardic responses were elicited by 10 mM concentration; therefore, this concentration of NMDA was selected for further studies in other groups of rats. The onset, peak and duration of the responses elicited by 10 mM concentration of NMDA were 5–20 sec, 1–2 min, and 10–15 min, respectively. In this and other series of experiments, microinjections of aCSF alone into the PVN and mNTS or its direct application to mNTS neurons did not elicit any responses (n = 4).
Table 1.
Concentration-response in the PVN (50 nl, n = 4, each group)
| A: NMDA | ||||
|---|---|---|---|---|
| 1 mM | 2.5 mM | 5 mM | 10 mM | |
| Baseline MAP | 93.8 ± 5.5 | 95.0 ± 4.1 | 92.5 ± 3.2 | 93.8 ± 4.7 |
| MAP response | 12.5 ± 2.5** | 21.3 ± 3.1* | 23.8 ± 2.4* | 30.0 ± 2.9 |
| Baseline HR | 425.0 ± 10.4 | 427.5 ± 7.5 | 422.5 ± 16.5 | 432.5 ± 11.1 |
| HR response | 11.3 ± 3.1* | 13.8 ± 2.4* | 20.0 ± 2.0* | 31.3 ± 3.1 |
| B: AMPA | ||||
|---|---|---|---|---|
| 1 mM | 2.5 mM | 5 mM | 10 mM | |
| Baseline MAP | 93.8 ± 4.7 | 98.8 ± 8.3 | 95.0 ± 5.4 | 96.3 ± 7.2 |
| MAP response | 7.5 ± 1.4** | 13.8 ± 2.4* | 15.0 ± 2.0* | 21.3 ± 2.3 |
| Baseline HR | 417.5 ± 15.5 | 422.5 ± 8.5 | 427.5 ± 7.5 | 425.0 ± 10.4 |
| HR response | 2.5 ± 1.4** | 5.0 ± 2.0* | 8.8 ± 1.3* | 17.5 ± 3.2 |
Values are expressed as mean ± SEM
P < 0.05
P < 0.01 compared to 10 mM
Concentration-response of AMPA in the PVN
Cardiovascular responses to unilateral microinjections of NMDA into the PVN were compared with a non-NMDA receptor agonist, AMPA. Unilateral microinjections of AMPA into the PVN elicited increases in MAP and HR (Table. 1B). Maximal pressor and tachycardic responses were elicited by 10 mM AMPA. The onset, peak and duration of AMPA (10 mM) were 5–20 sec, 1–3 min, 15–20 min, respectively.
The cardiovascular responses to unilateral microinjections of NMDA into the PVN were generally greater than those of AMPA. For example, the pressor responses to 10 mM of NMDA were significantly greater (P < 0.05) than those elicited by AMPA. Similarly, the tachycardic responses to the same concentrations of NMDA were significantly greater (P < 0.05) than those elicited by AMPA.
Reproducibility of NMDA responses in the PVN
The concentration of NMDA that elicited maximal cardiovascular responses was microinjected into the PVN at least 3 times at 20 min intervals. No tachyphylaxis was observed in cardiovascular responses to repeated microinjections of NMDA (Table 2). The concentrations of NMDA (10 mM) that elicited cardiovascular responses when microinjected into the PVN did not elicit a response when administered intravenously (n = 4).
Table 2.
Reproducibility of NMDA (10 mM, 50 nl) responses in the PVN (n = 4)
| Microinjection of NMDA | 1 st | 2 nd | 3 rd |
|---|---|---|---|
| Baseline MAP | 95.0 ± 8.4 | 96.3 ± 5.2 | 93.8 ± 4.3 |
| MAP response | 30.0 ± 3.5 | 28.8 ± 2.3 | 28.8 ± 1.3 |
| Baseline HR | 435.0 ± 6.5 | 430.0 ± 8.2 | 430.0 ± 7.1 |
| HR response | 27.5 ± 3.2 | 26.3 ± 2.4 | 26.3 ± 3.8 |
Values are expressed as mean ± SEM
Beta adrenergic receptor blockade: effect on PVN responses
The effect of blockade of cardiac beta-adrenergic receptors on NMDA-induced HR responses was tested in another group of rats. Twenty min after the unilateral microinjection of NMDA into the PVN, atenolol was injected intravenously; the bolus injection of atenolol alone elicited a decrease in HR (67.5 ± 7.5 bpm) and a slight increase in MAP (3.8 ± 2.4 mmHg). When the HR returned to about 80% of baseline values (about 25 min), NMDA was again microinjected into the PVN. The tachycardic response induced by the microinjection of NMDA into the PVN was significantly attenuated by atenolol (Table 3).
Table 3.
Beta adrenergic receptor blockade (atenolol 75 µg/kg i.v.): effect on NMDA (10 mM, 50 nl) response in the PVN (n = 4)
| Before atenolol | After atenolol | |
|---|---|---|
| Baseline MAP | 92.5 ± 6.6 | 94.3 ± 8.3 |
| MAP response | 28.8 ± 3.1 | 30.0 ± 5.0 |
| Baseline HR | 425.0 ± 9.6 | 411.3 ± 10.1* |
| HR response | 32.5 ± 4.3 | 17.5 ± 4.8* |
Values are expressed as mean ± SEM
P < 0.05, compared to the value before atenolol
Exaggeration of PVN responses by glutamate receptor blockade in the mNTS
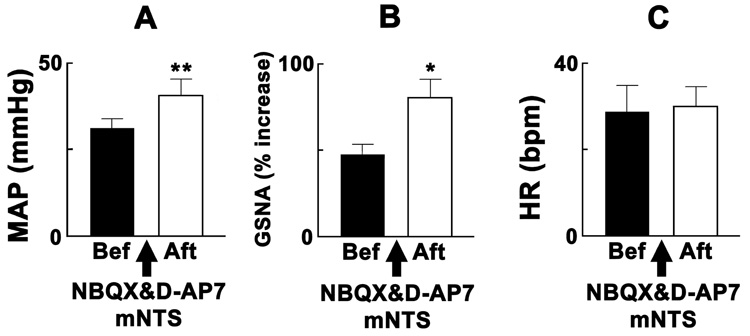
Group data for the effects of the bilateral blockade of ionotropic glutamate receptors in the mNTS on the responses elicited by the chemical stimulation of the PVN are shown in Fig. 1. Chemical stimulation of the PVN was always induced by unilateral microinjections of NMDA. The pressor (Fig. 1A) and GSNA (Fig. 1B) responses elicited by the PVN stimulation were significantly exaggerated by bilateral microinjections of NBQX and D-AP7, microinjected sequentially within 1 min, into the mNTS. The interval between the microinjections of the ionotropic glutamate receptor antagonists into the mNTS and NMDA into the PVN was 10 min. HR responses to the PVN stimulation were not significantly altered (Fig. 1C). Bilateral microinjections of aCSF into the mNTS did not alter the responses to the chemical stimulation of the PVN (n = 4).
Fig. 1.
Exaggeration of PVN responses by blockade of glutamate receptors in the mNTS. The rats were barodenervated. Black and open bars: responses to unilateral microinjections of NMDA (10 mM, 50 nl) into the PVN before and after, respectively, the bilateral microinjections of NBQX (2 mM, 50 nl) and D-AP7 (5 mM, 50 nl) into the mNTS (n = 8). A: The increases in MAP elicited by the PVN stimulation before and after the blockade of ionotropic glutamate receptors in the mNTS were 31.3 ± 2.6 and 40.6 ± 4.8 mmHg, respectively. B: The increases in the greater splanchnic nerve activity (GSNA) before and after the same treatment were 47.1 ± 6.9 and 80.3 ± 10.7 %, respectively. C: The increases in HR before and after the same treatment were 28.8 ± 6.1 and 30.0 ± 4.6 bpm, respectively. Baseline MAP before and after the bilateral microinjections of ionotropic glutamate receptor antagonists into the mNTS were 85.6 ± 4.0 and 91.9 ± 4.6 mmHg, respectively (P > 0.05). Baseline HR was 428.8 ± 12.7 and 425.0 ± 13.5 bpm, respectively (P > 0.05). *P < 0.05, **P < 0.01 compared with the values before the microinjections of ionotropic glutamate receptor antagonists into the mNTS.
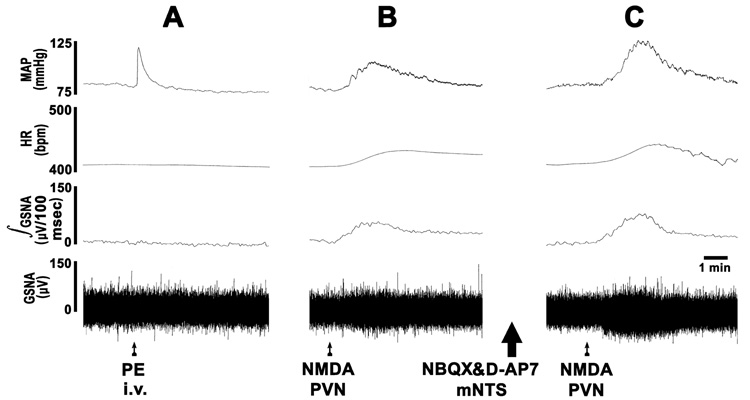
The tracings showing the effects of the bilateral blockade of ionotropic glutamate receptors in the mNTS on the responses induced by the PVN stimulation are presented in Fig. 2. After barodenervation, a bolus injection of phenylephrine elicited a rapid pressor response but no reflex decrease in HR or GSNA indicating that the barodenervation was complete (Fig. 2A). Subsequently, a unilateral microinjection of NMDA into the PVN elicited an increase in MAP, HR and GSNA (Fig. 2B). Twenty min later, the mNTS was identified bilaterally by microinjections of L-Glu (5 mM; not shown). After an interval of 5 min, NBQX and D-AP7 were microinjected bilaterally into the same mNTS sites (tracings not shown). After an interval of 10 min, NMDA was again microinjected unilaterally into the PVN; exaggerated increases in MAP and GSNA were elicited (Fig. 2C).
Fig. 2.
Typical tracings of the results shown in Fig. 2. Top trace: MAP, 2nd trace: HR, 3rd trace: integrated GSNA (µV/100 msec), bottom trace: raw nerve activity (µV). A: bolus injection of phenylephrine (PE, 4 µg/kg, i.v.) did not elicit reflex bradycardia and inhibition of GSNA indicating that barodenervation was complete. B: Subsequently, unilateral microinjection of NMDA (10 mM, 50 nl) into the PVN elicited an increase in MAP, HR and GSNA. After an interval of 20 min, NBQX (2 mM, 50 nl) and D-AP7 (5 mM, 50 nl) were microinjected bilaterally into the previously identified mNTS (large arrow); increases in MAP and GSNA were elicited (tracing not shown). C: After an interval of 10 min, when the MAP and GSNA recovered to about 80% of the baseline values, NMDA (10 mM, 50 nl) was again microinjected into the same site in the PVN; exaggerated increases in MAP and GSNA were elicited.
Since elimination of baroreceptor afferents can alter the cardiovascular control mechanisms, the results were confirmed in intact rats. The results obtained in intact rats were qualitatively identical to those obtained in barodenervated rats. Like the responses in barodenervated rats, the increase in MAP elicited by microinjections of NMDA (10 mM, 50 nl) into the PVN in intact rats (n = 5) was significantly (P < 0.05) exaggerated after the bilateral microinjections of NBQX (2 mM, 50 nl) and D-AP7 (5 mM, 50 nl) into the mNTS; the increases in MAP in response to the PVN stimulation were 21.0 ± 2.9 and 43.0 ± 5.6 mmHg, before and after the glutamate receptor blockade in the mNTS.
NTS-neuronal responses to the PVN stimulation
The responses of mNTS neurons to chemical stimulation of PVN by microinjections of NMDA were studied in 10 rats. A smaller volume of microinjection of NMDA (20 nl) into the PVN was used in this experiment in order to reduce the chances of losing the recorded neurons due to large increases in BP. A total of 61 neurons were recorded. Fourteen of these neurons did not respond to electrical stimulation of the ipsilateral AN (50–100 µA, 0.1 msec, 1 Hz). The maximum firing rate of these neurons before and after AN stimulation was 5.1 ± 1.0 and 5.9 ± 1.2 spikes/sec, respectively. Fifteen of these neurons were inhibited by AN stimulation; the maximum firing rate of these neurons before and after AN stimulation was 5.4 ± 0.2 and 2.0 ± 0.4 spikes/sec, respectively. Thirty two of these neurons responding to AN stimulation increased their firing rates significantly (P < 0.001) from 5.0 ± 1.0 to 13.6 ± 1.7 spikes/sec. Twenty two out of the 32 neurons (69%) were also excited by chemical stimulation of the ipsilateral PVN (NMDA 10 mM, 20 nl); the firing rate significantly (P < 0.001) increased from the basal rate of 6.7 ± 1.2 to 16.0 ± 1.3 spikes/sec. Three neurons out of these 32 neurons (9%) were inhibited and 7 neurons (22%) exhibited no response to ipsilateral chemical stimulation of the PVN. All the recorded neurons were excited by direct application of L-Glu (5 mM, 4 nl); the firing rate significantly (P < 0.001) increased from the basal rate of 6.1 ± 1.0 to 15.3 ± 1.4 spikes/sec. We did not analyze the effects of PVN stimulation on the neurons that were either non-responsive to AN stimulation or were inhibited by it.
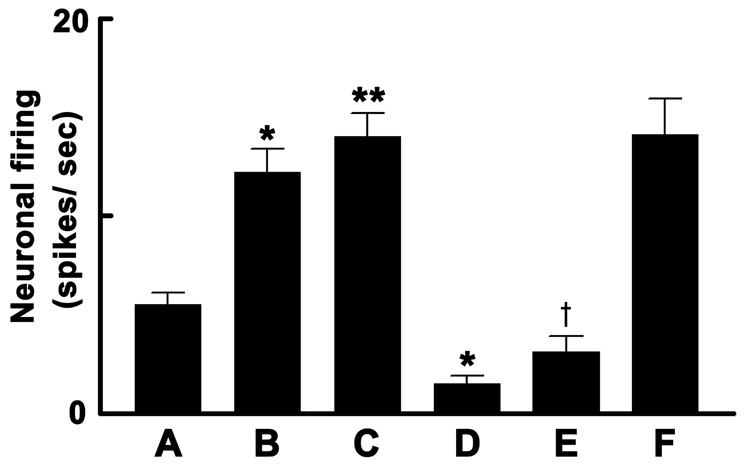
The effect of ionotropic glutamate receptor blockade on PVN-induced effects on mNTS neurons was studied in another group of rats. The basal firing rate of these neurons is shown in Fig. 3A. The firing increased significantly by direct application of L-Glu (Fig. 3B). Subsequently, ipsilateral AN stimulation increased the neuronal firing. Microinjection of NMDA into the ipsilateral PVN significantly increased the firing of these neurons (Fig. 3C). After the neuronal firing returned to basal values (within 5 min), NBQX and D-AP7 were directly applied to these neurons; the neuronal firing rate was significantly reduced by the application of these two antagonists (Fig. 3D). Five to ten sec later, NMDA was again microinjected into the ipsilateral PVN; the increase in the firing rate of mNTS neurons was significantly reduced (Fig. 3E) compared to the previous NMDA response in the PVN (see Fig. 3C). When NMDA was again microinjected into the PVN after 5 min, the response to the microinjection of NMDA recovered (Fig. 3F).
Fig. 3.
NTS-neuronal responses to PVN stimulation in barodenervated rats. A: basal firing rate of mNTS neurons was 5.5 ± 0.6 spikes/sec (n = 5, 12 neurons). B: Direct application of L-Glu (5 mM, 4 nl) increased the neuronal firing to 12.2 ± 1.2 spikes/sec. Subsequently, ipsilateral aortic nerve stimulation (50–100 µA, 0.1 msec, 1 Hz) increased the neuronal firing (data not shown). C: Chemical stimulation of ipsilateral PVN by NMDA microinjection (10 mM; 20 nl) increased the neuronal firing to 14.0 ± 1.2 spikes/sec. D: Direct application of NBQX (2 mM, 4 nl) and D-AP7 (5 mM, 4 nl) (applied sequentially within 2–3 sec) decreased the neuronal firing to 1.5 ± 0.4 spikes/sec. E: Excitation of the neuron due to unilateral PVN stimulation by NMDA was attenuated after ionotropic glutamate receptor blockade in the mNTS; the increase in firing was 3.1 ± 0.8 spikes/sec. F: Excitation of the neuron induced by PVN stimulation recovered to 14.1 ± 1.8 spikes/sec after 5 min; compare F with C (P > 0.05). Baseline MAP before and after the unilateral microinjections of ionotropic glutamate receptor antagonists into the mNTS was 93.6 ± 3.2 and 92.3 ± 2.1 mmHg, respectively (P > 0.05). Baseline HR was 430.2 ± 4.5 and 428.8 ± 7.4 bpm, respectively (P > 0.05). *P < 0.01 (compare with bar A); **P < 0.001 (compare with bar A); †P < 0.001 (compare with bar C).
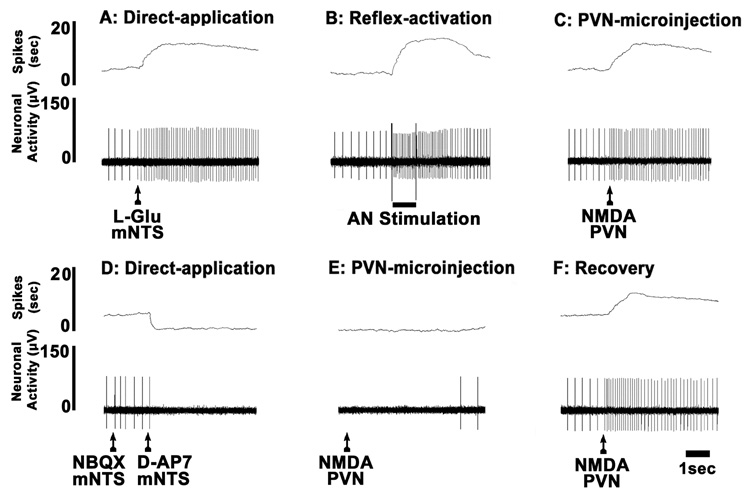
A typical tracing of the neuronal recording results is shown in Fig. 4. Direct application of L-Glu onto the mNTS neuron increased its firing (Fig. 4A). After the neuronal firing returned to the basal level, ipsilateral AN stimulation elicited an increase in the neuronal firing indicating that this neuron was barosensitive (Fig. 4B). Within an interval of 1 min, microinjection of NMDA into the ipsilateral PVN increased the neuronal firing (Fig. 4C). Five min later, direct application of NBQX and D-AP7 to the mNTS neuron inhibited its firing (Fig. 4D). Within 10 sec, while the ionotropic glutamate receptors on the mNTS neurons were blocked, NMDA was again microinjected into the ipsilateral PVN; no increase in neuronal activity was observed (Fig. 4E). The response to the microinjection of NMDA into the PVN showed recovery after 5 min (Fig. 4F).
Fig. 4.
A typical tracing of extracellular neuronal recording in a barodenervated rat. The firing of the mNTS neuron was increased by direct application of L-Glu (5 mM, 4 nl) (A), electrical stimulation of the ipsilateral aortic nerve (B), and microinjection of NMDA (10 mM, 20 nl) into the ipsilateral PVN (C). Direct application of NBQX (2 mM, 4 nl) and D-AP7 (5 mM, 4 nl) abolished the neuronal firing (D). Within 10 sec, NMDA (10 mM, 20 nl) was again microinjected into the PVN; no excitation was observed due to ionotropic glutamate receptor blockade of the mNTS neuron (E; compare with C). After 5 min, NMDA (10 mM, 20 nl) was again microinjected into the PVN; the excitation of the neuron resumed (F, compare with C).
The excitatory projection from the PVN to the mNTS is tonically active
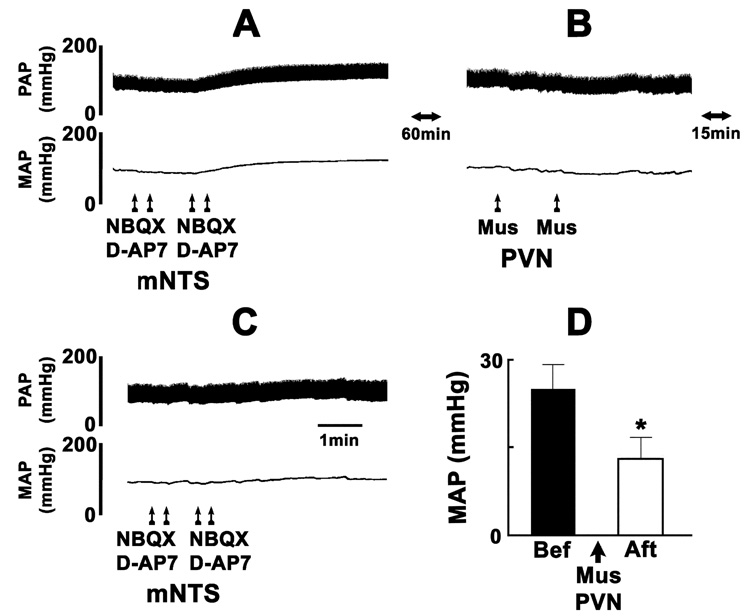
In another group of rats, experiments were done to test if the excitatory projection from the PVN to the mNTS is tonically active. Barodenervation in this group of rats increased the MAP (38.0 ± 3.4 mmHg) and HR (44.0 ± 5.1 bpm) which lasted for about 60 min. A typical tracing of the results of these experiments is shown in Fig. 5. Bilateral microinjections of NBQX and D-AP7 into the mNTS resulted in increases in MAP (Fig. 5A) which lasted for 10–15 min. After an interval of 60 min, bilateral microinjections of muscimol into the PVN elicited a small decrease in MAP (Fig. 5B). Fifteen min later, when the MAP returned to about 80% of baseline values, NBQX and D-AP7 were again microinjected bilaterally into the mNTS; the increase in MAP was attenuated (Fig. 5C). The purpose of waiting for 15 min after the microinjection of muscimol into the mNTS was to allow the BP to recover close to the basal levels so that the attenuated responses to microinjections of NBQX and D-AP7 into the mNTS could not be attributed to lower basal levels of MAP. Group data of these experiments are shown in Fig. 5D. A significant attenuation of the increase in BP induced by bilateral blockade of ionotropic glutamate receptors in the mNTS was observed after the bilateral inhibition of PVN neurons by muscimol. These results indicated that the mNTS received a tonically active excitatory input from the PVN.
Fig. 5.
The projection from the PVN to the mNTS is tonically active. A typical tracing from a barodenervated rat. Top trace: pulsatile arterial pressure (PAP), bottom trace: mean arterial pressure (MAP). A: bilateral microinjections of NBQX (2 mM, 50 nl) and D-AP7 (5 mM, 50 nl) into the mNTS increased MAP. B: After 60 min, muscimol (Mus, 0.5 mM, 50 nl) was bilaterally microinjected into the PVN; a small decrease (15 ± 3.5 mmHg) in MAP was elicited. C: Fifteen min later, MAP recovered to about 80% of the baseline values. At this time, the increases in MAP to bilateral microinjections of NBQX and D-AP7 into the mNTS were attenuated. D: Group data (n = 5). Increases in MAP elicited by bilateral microinjections of NBQX and D-AP7 into the mNTS before and after bilateral microinjections of muscimol into the PVN were 25.0 ± 4.2 mmHg (black bar) and 13.0 ± 3.7 mmHg (open bar), respectively. Baseline MAP values before and after the microinjections of muscimol into the PVN were 91.0 ± 4.3 and 87.0 ± 5.1 mmHg, respectively (P > 0.05). *P < 0.01 compared with black bar.
In another group of rats (n = 4), bilateral vagotomy was performed low in the neck in addition to bilateral barodenervation. The increase in MAP (25.0 ± 3.5 mmHg) induced by bilateral microinjections of D-AP7 and NBQX in these rats was identical to that observed in the rats with bilateral barodenervation without vagotomy (25.0 ± 4.2 mmHg).
In other experiments (n = 4), bilateral microinjections of NBQX (2 mM, 50 nl) and D-AP7 (5 mM, 50 nl) into the mNTS, repeated at least 2 times, at 60 min intervals, did not exhibit tachyphylaxis; the increases in MAP in response to the first and second microinjection of these antagonists were 22.5 ± 3.2 and 21.3 ± 2.4 mmHg, respectively (P > 0.05; n = 4). Since the HR change elicited by the bilateral blockade of ionotropic glutamate receptor in the mNTS were generally small (< ± 10 bpm), these changes were not analyzed in this experiment.
Retrograde tracing of PVN projections
Unilateral microinjection of fluorogold into the mNTS resulted in retrograde labeling in the ipsilateral (Fig. 6 A&B) as well as contralateral (Fig. 6 C&D) PVN neurons. Examination of the sections showed that the labeling exhibited ipsilateral preponderance. The site of microinjection of fluorogold into the mNTS on one side is shown in Fig. 6E.
Fig. 6.
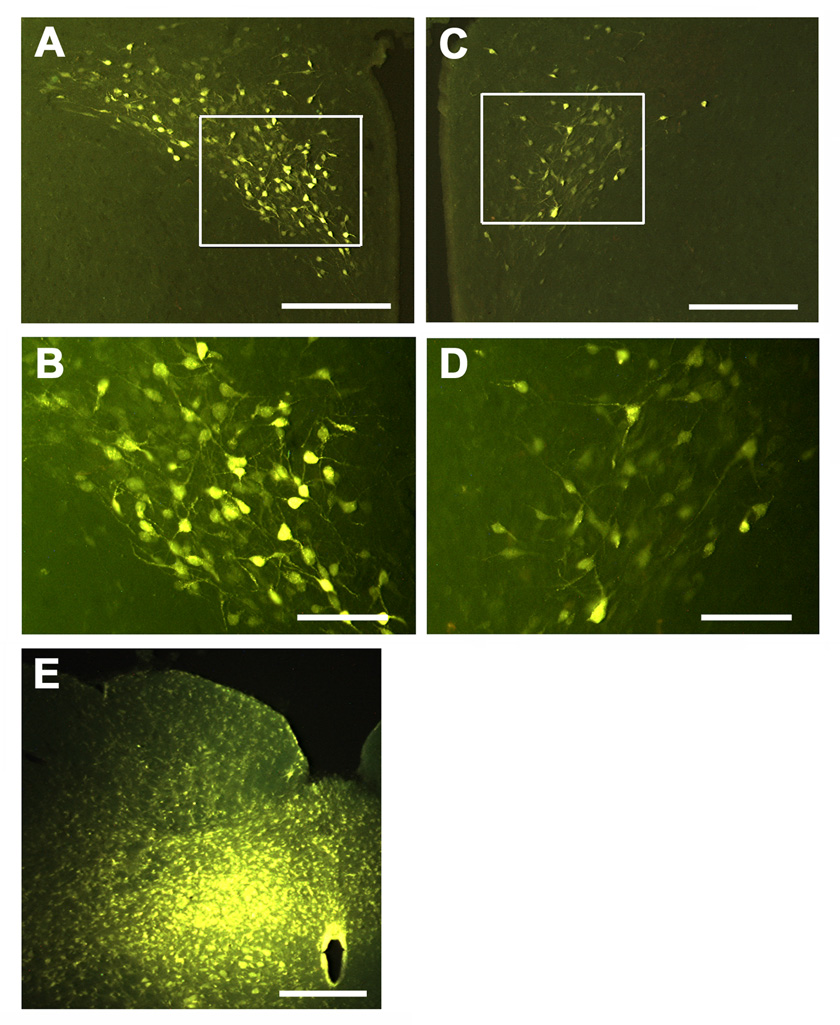
Retrograde labeling of PVN neurons. A, C and E = low magnification. B and D high magnification of boxed areas in A and C, respectively. Labeling of ipsilateral (A) and contralateral (C) PVN following unilateral microinjection (15 nl) of fluorogold (4–6%) into the mNTS (E). Examination of the section shows preponderance of the labeling in the ipsilateral PVN (A and B). Scale bars: 300 µm in A and C; 100 µm in B and D; 400 µm in E.
Anterograde tracing of PVN projections and immunohistochemistry for VGLUT2
Unilateral microinjection of BDA into the PVN resulted in anterograde labeling in the ipsilateral as well as contralateral mNTS; the labeling was predominant in the ipsilateral mNTS. A cluster of BDA-labeled terminals in mNTS is shown in Fig. 7A. VGLUT2 immunoreactivity in the same field is shown in Fig. 7B. A merged image shown in Fig. 7C indicated that the BDA-labeled terminal was VGLUT2-immunoreactive. The site of microinjection of BDA in the PVN is shown in Fig. 7D. Similar techniques have been used to identify a glutamatergic projection from the PVN to the RVLM (Stocker et al., 2006).
Fig. 7.
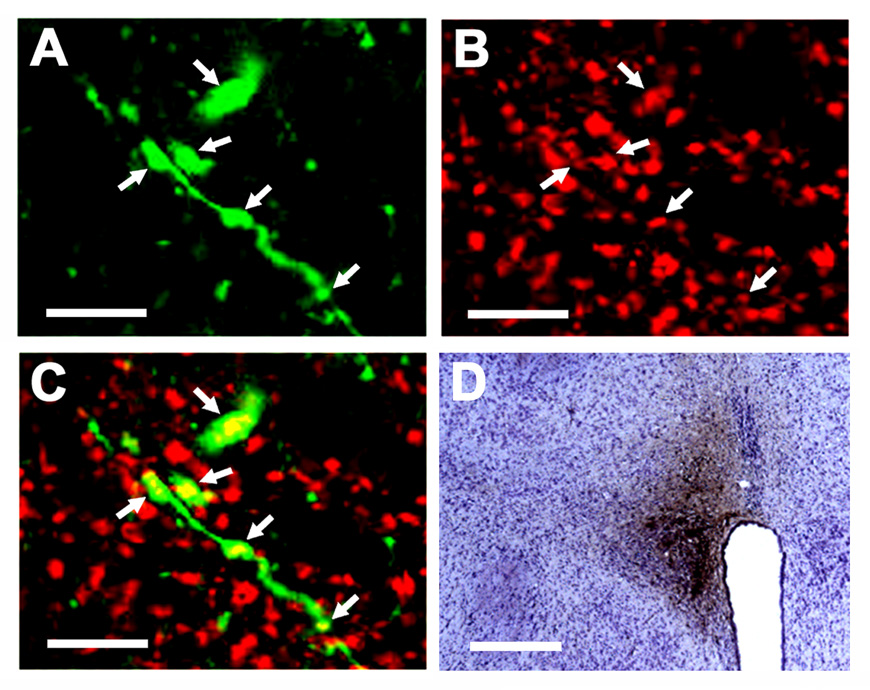
Immunoreactivity of vesicular glutamate transporter-2 (VGLUT2) in the terminals of PVN neurons innervating the mNTS. A cluster of BDA-labeled axons and terminals (green) is shown in panel A (confocal microscopy; Alexa Fluor 488 fluorescence). VGLUT2 immunoreactivity (red) in the same field is shown in panel B (Cy3 fluorescence). A merged image shown in panel C indicates that the BDA-labeled terminals are VGLUT2-immunoreactive (colocalization is shown by yellow color). The site of microinjection of BDA (10%, 100 nl) in the PVN is shown in panel D. Scale bars; 20 µm (panels A, B, C) and 300 µm (panel D).
Histological identification of microinjection sites
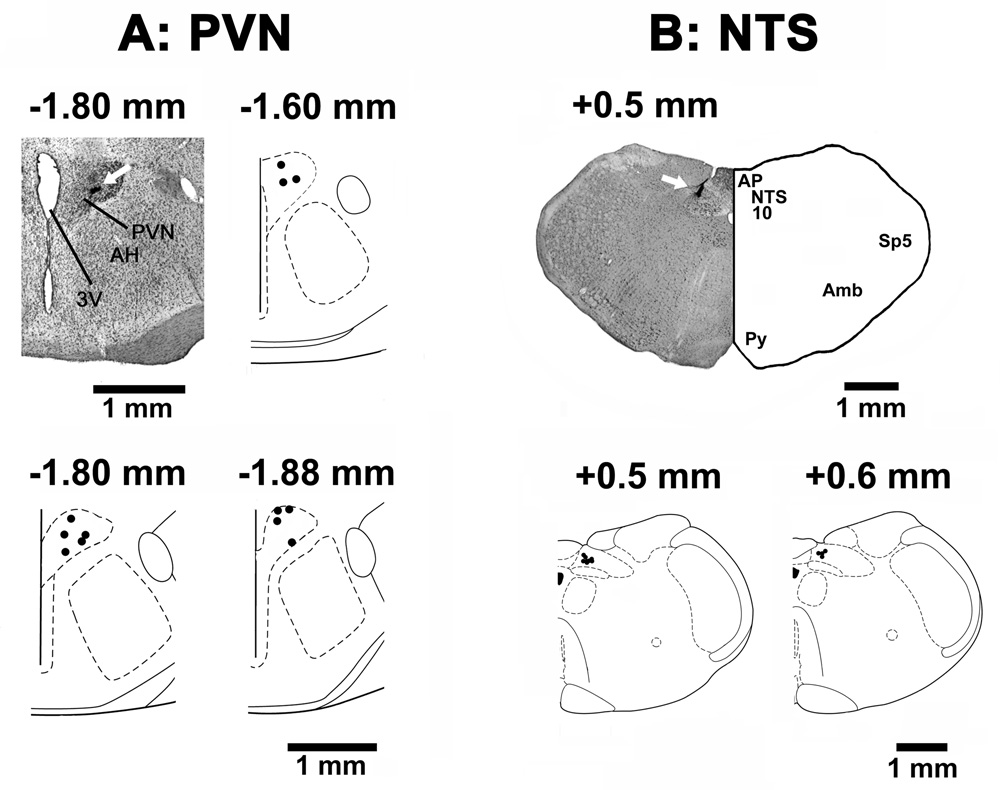
The PVN sites, where microinjections of NMDA elicited excitatory effects, were marked with India ink in 14 rats. A typical PVN site and composite diagrams of the PVN microinjection sites are shown in Fig. 8A. The mNTS sites, where NBQX and D-AP7 were microinjected, were marked in 12 rats. A typical microinjection site in the mNTS and composite diagrams are presented in Fig. 8B.
Fig. 8.
Histological identification of microinjection sites. A: A coronal section at a level 1.8 mm caudal to the bregma showing a typical PVN microinjection site marked (arrow) with India ink; the center of the spot was 0.5 mm lateral to the midline and 7.8 mm deep from the dura. Composite diagrams in this panel represent the PVN sites (n = 12), where NMDA was microinjected, at levels 1.60, 1.8 and 1.88 mm caudal to the bregma. B: A coronal section at a level 0.5 mm rostral to the calamus scriptorius showing a typical mNTS microinjection site marked (arrow) with India ink; the center of the spot was 0.5 mm lateral to the midline and 0.5 mm deep from the dorsal medullary surface. Composite diagrams in this panel represent the mNTS sites (n = 12) at levels 0.5 and 0.6 mm rostral to the calamus scriptorius. In the composite diagrams, each spot represents the microinjection site in one rat; all spots are not visible due to some overlap. Abbreviations: AH: anterior hypothalamic nucleus; Amb: nucleus ambiguus, AP: area postrema, NTS: nucleus tractus solitarius; PVN: hypothalamic paraventricular nucleus; Py: pyramids; Sp5: spinal trigeminal tract; 3V: 3rd ventricle; 10: dorsal motor nucleus of vagus.
DISCUSSION
The following new observations were made in this study: 1) a glutamatergic bilateral projection from the PVN to the mNTS was identified, 2) bilateral blockade of ionotropic glutamate receptors in the mNTS exaggerated the pressor and sympathetic nerve responses to unilateral chemical stimulation of the PVN, and 3) the projection from the PVN to the mNTS was determined to be tonically active.
NMDA has been used to stimulate PVN in only a few investigations (Li et al., 2001 and 2006). In these reports (Li et al., 2001 and 2006), a push-pull perfusion cannula was placed in the PVN and different volumes (25–200 nl) of the same concentration (1 mM) of NMDA were used to estimate concentration-response relationship. There are no reports in which AMPA has been used to stimulate the PVN. Therefore, concentration-response relationships of NMDA and AMPA in the PVN were studied systematically. Chemical stimulation of the PVN by unilateral microinjections of NMDA elicited increases in BP that were mediated via an increase in efferent sympathetic nerve activity. The increase in HR was also partly mediated via sympathetic activation because blockade of beta-1 adrenergic receptors by atenolol attenuated tachycardia. Activation of non-NMDA receptors in the PVN by unilateral microinjections of AMPA also elicited pressor and tachycardic responses but these responses were generally smaller than those elicited by the same concentration of NMDA. The responses to unilateral microinjections of excitatory amino acids into the PVN showed concentration-dependent increments.
NMDA concentrations used to stimulate the PVN in this and other laboratories (Li et al., 2006) are generally higher than those needed for stimulating medullary cardiovascular areas (Dhruva et al., 1998). The reason for the necessity to use higher concentrations of L-Glu and NMDA to stimulate the PVN may be that the PVN neurons are tonically inhibited by GABA (Li et al., 2006), or nitric oxide (Stern, 2004). Another possibility is that the injected material in the PVN is dissipated relatively faster because this nucleus is one of the most highly vascularized regions of the brain; the capillary density in the PVN is 2000 mm/mm3 (Rivest, 2002).
L-Glu has also been used for chemical stimulation of the PVN; however, the concentrations needed to stimulate the PVN chemically have been reported to be high (0.5–1 M) (Darlington et al., 1989; Katafuchi et al., 1988; Martin and Haywood, 1992; Yamashita et al., 1987).
The concentrations of NMDA that elicited pressor and tachycardic responses when microinjected into the PVN (i.e., 10 mM, 50 nl), did not elicit a response when injected intravenously indicating that leakage of the drug, if any, from the microinjection site in the PVN to the peripheral circulation was not responsible for the observed responses. In concentration-response and other studies, microinjections of aCSF alone did not elicit any responses indicating that any non-specific effects, such as distortion of the brain tissue due to microinjections, did not contribute to the responses.
Anesthetics are known to affect cardiovascular system. For example, combination of urethane and alpha-chloralose has been reported to decrease baseline BP, HR and mesenteric resistance, increase plasma renin activity, and induce a vasopressin-dependent vascular tone while the baroreflex slope for pressor and depressor stimuli was not altered (Faber, 1989). Obviously these effects of anesthetics may have contributed to some of the responses observed in this study. For example, chemical stimulation of the PVN may have resulted in the release of vasopressin into the circulation and exaggerated the anesthetic-induced vasopressin-dependent vascular tone. Moreover, patch-clamp experiments on brain slice preparations have shown that urethane decreased the frequency of GABAergic, but not glutamatergic, spontaneous post-synaptic currents (Accorsi-Mendonca et al., 2007). In our study, it is possible that the tone of GABAergic inputs to the PVN (Li et al., 2006) is decreased by the anesthetic and the PVN neurons are rendered more excitable. However, the use of an anesthetized preparation was necessary in this study because of the invasive procedures involved. This preparation does have some advantages. For example, discrete regions of the brain (in this case PVN and mNTS) can be identified and pharmacologically manipulated. The secondary effects, such as those due to respiratory changes, can be avoided by artificial ventilation; this procedure cannot be applied to conscious animals. Moreover, the effects of stress can be reduced, if not eliminated, in anesthetized preparations. Each animal served as its own control in most of our experiments. Therefore, the observed changes in PVN responses by pharmacological manipulations in the mNTS could not be ascribed to the anesthetic effects.
Acute barodenervation resulted in increases in BP and HR which returned to basal levels within 60 min. The recovery of BP and HR has been attributed to the failure to maintain sustained elevation of sympathetic nerve activity (Osborn and England, 1990).
In barodenervated rats, blockade of ionotropic glutamate receptors in the mNTS, by bilateral microinjections of NBQX and D-AP7, resulted in exaggerated increases in BP and GSNA in response to unilateral microinjections of NMDA into the PVN. In order to avoid the effect of any alterations in the cardiovascular control mechanism that elimination of baroreceptor and other afferents may have caused, the experiments were repeated in intact rats. The results obtained in intact rats were qualitatively identical to those obtained in barodenervated rats. The increases in BP and sympathetic nerve excitation to chemical stimulation of PVN may be mediated via the well established excitatory projection from the PVN to the RVLM (Yang and Coote, 1998). Normally, the excitatory cardiovascular effects of this pathway (i.e., from the PVN to RVLM) may be restrained by the inhibitory effects of the NTS on the RVLM that are exerted via the CVLM (Sapru, 2002). Our conclusion that excitatory effect of the PVN to RVLM pathway is restrained by the inhibitory effect of the NTS on the RVLM via the CVLM is based on the observation that the blockade of ionotropic glutamate receptors in the mNTS resulted in exaggerated responses to PVN stimulation. This observation also indicated that the projection from the PVN to the mNTS utilizes an excitatory amino acid (probably glutamate) as a neurotransmitter that mediates its actions via ionotropic glutamate receptors. It may be argued that microinjections of ionotropic glutamate receptor antagonists may alter the responses of NTS neurons to other neurotransmitters. This possibility is unlikely because we have previously shown that the effects of D-AP7 and NBQX are specific for ionotropic glutamate receptors. For example, these antagonists did not alter the responses to other agonists such as carbachol (Kasamatsu et al, 2004) or metabotropic glutamate receptor agonists (Viard and Sapru, 2002).
As mentioned in the Introduction, the PVN sends vasopressinergic or oxytocinergic projections to the mNTS (Buijs, 1978; Palkovits, 1999; Sofroniew and Schrell, 1981) and these projections have been reported to be involved in cardiovascular regulation at rest and exercise (Braga et al., 2000; Dufloth et al., 1997; Higa et al., 2002; Michelini, 2007). Vasopressin has been reported to cause presynaptic inhibition of terminal glutamate release in brainstem slices (Bailey et al., 2006) and unilateral microinjections of vasopressin or oxytocin into the mNTS have been reported to increase BP and HR (Matsuguchi et al., 1982). Based on these reports (Bailey et al., 2006; Braga et al. 2000; Dufloth et al., 1997; Higa et al., 2002; Michelini, 2007) and the results of our study, it is conceivable that the peptidergic projections together with the glutamatergic projections from the PVN to the mNTS may be involved in modulation and integration of cardiovascular function via a complex interaction with GABAergic and glutamatergic mechanisms in the NTS.
Based on earlier reports (Sapru, 2002; Talman et al., 1984), the release of glutamate in the mNTS following the PVN stimulation should decrease BP and HR. In this context, it should be noted that unilateral microinjections of NMDA into the PVN stimulate the projections from the PVN to the RVLM and mNTS simultaneously. The stimulation of the projection to the RVLM elicits pressor and tachycardic responses while the projection to the mNTS elicits opposite responses. However, the projection from the PVN to the RVLM predominates and the net effect is pressor and tachycardic responses. It should be noted that baroreceptor afferents are already denervated in these rats, therefore, blockade of baroreflex at the level of mNTS by bilateral microinjections of NBQX and D-AP7 could not have accounted for the exaggeration of PVN responses.
Our anatomic studies support our conclusion that a direct glutamatergic projection from the PVN to the mNTS exists. This conclusion is based on the following observations: 1) unilateral microinjections of fluorogold into the mNTS retrogradely labeled the PVN neurons bilaterally, with ipsilateral preponderance, 2) unilateral microinjections of BDA into the PVN anterogradely labeled terminals in the mNTS bilaterally with ipsilateral predominance, and 3) the anterogradely labeled terminals of PVN neurons in the mNTS exhibited immunoreactivity for VGLUT2. Published literature indicates that VGLUT2 is located within terminals that form asymmetric (excitatory) synapses (Herzog et al., 2001). Our conclusion that there is a direct projection from the PVN to the NTS is in agreement with earlier reports (Hardy, 2001; Luiten et al., 1985). That the projections from the PVN to the NTS are monosynaptic is further supported by the report that PVN neurons are antidromically activated by the electrical stimulation of the NTS (Kannan and Yamashita, 1983). We have extended the results of these anatomical studies (Hardy, 2001; Luiten et al., 1985) by demonstrating immunohistochemically for the first time that the projections from the PVN to the mNTS are glutamatergic.
Our observations using microinjection technique were confirmed by extracellular recording of single mNTS neurons. In our experiments, excitation of the NTS neurons by direct applications of L-Glu indicated that the activity was recorded from neuronal cell bodies. The participation of the recorded NTS neurons in baroreflex was indicated by their excitation in response to the electrical stimulation of the ipsilateral aortic depressor nerve which contains primarily baroreceptor afferents (Sapru et al., 1981). These rats were barodenervated; therefore, intravenous phenylephrine could not be used to test the barosensitivity of the neuron. The latencies of the neuronal excitatory responses to the AN stimulation were not measured. Therefore, we cannot be sure whether we were recording from monosynaptic or polysynaptic neurons that have been described by Scheuer et al. (1996). Chemical stimulation of the PVN elicited either excitation (69%), or inhibition (9%) or no response (22%) of ipsilateral NTS neurons. Similar neuronal responses to the PVN stimulation have been reported by others, however, the percentages of NTS neuronal populations excited or inhibited by the PVN stimulation were different in these reports compared to our results. For example, Kannan and Yamashita (1985) reported PVN-induced excitation of 8.6% of barosensitive NTS neurons in anesthetized rats. On the other hand, Duan et al (1999) reported PVN-induced excitation in 23.5%, inhibition in 52.9% and no responses in 23.5% of barosensitive NTS neurons in anesthetized rabbits. The reasons for the differences in the proportion of NTS neurons excited or inhibited by PVN stimulation in our experiments and those of others may be that in these studies (Duan et al., 1999; Kannan and Yamashita, 1985), the PVN was stimulated electrically which may have resulted in the activation of fibers of passage from regions other than PVN. Blockade of excitatory amino acid (EAA) receptors on barosensitive NTS neurons results in either silencing or inhibition of these neurons. This observation is in agreement with earlier reports (Zhang and Mifflin, 1998). The new finding in our neuronal recording experiments is that the excitation of mNTS neurons induced by the chemical stimulation of the ipsilateral PVN was prevented by prior direct applications of NBQX and D-AP7 to these neurons indicating that the mNTS neurons receive a glutamatergic input from the PVN neurons. These mNTS neurons were excited by activation of ipsilateral baroreceptor afferents.
Another important observation in our experiments is that bilateral microinjections of NBQX and D-AP7 into the mNTS increased BP indicating that there is a tonically active glutamatergic input to the mNTS. Since this response was observed in bilaterally barodenervated and vagotomized rats, the source of this tonically active input must be other than these afferents. Our results suggest that one of the sources of this input may be the PVN because bilateral inhibition of PVN by muscimol microinjections significantly attenuated the increase in BP induced by bilateral microinjections of NBQX and D-AP7 into the mNTS. The effect of muscimol in the PVN persisted, despite the recovery of MAP to near basal levels, because microinjections of NMDA into the same site in the PVN where muscimol was injected was attenuated after 20–25 min; the responses to NMDA re-appeared 30–40 min after the microinjection of muscimol. In other studies also, the effects of muscimol have been reported to last about 40 min (Willette et al., 1983). The recovery of MAP to near basal levels (within 15 min), despite the persistent effects of muscimol in the PVN, may be ascribed to the compensatory effects of other cardiovascular regulatory areas.
CONCLUSION
In summary, using anatomical, physiological and electrophysiological techniques, we have demonstrated the presence of a glutamatergic projection from the PVN to the mNTS. The projection is bilateral with ipsilateral preponderance. Since the blockade of this projection in the mNTS exaggerated the pressor responses to the PVN stimulation, it may be concluded that its normal function is to restrain the excitatory cardiovascular effects of PVN stimulation. The projection from the PVN to the mNTS is tonically active.
Acknowledgments
This work was supported in part by N.I.H. grants HL024347 and HL076248 awarded to Dr. H. N. Sapru
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AMPA
(±)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AN
aortic nerve
- BDA
dextran, biotin
- BP
blood pressure
- bpm
beats/min
- CVLM
caudal ventrolateral medullary depressor area
- D-AP7
D(−)-2-amino-7-phosphono-heptanoic acid
- GSNA
greater splanchnic nerve activity
- HR
heart rate
- L-Glu
L-glutamate
- MAP
mean arterial pressure
- mNTS
medial subnucleus of the nucleus tractus solitarius
- NBQX disodium salt
2,3-dioxo-6-nitro-1,2,3,4-tetrahydro-benzo[f]quinoxaline-7-sulfonamide disodium
- NMDA
N-methyl-D-aspartic acid
- NTS
nucleus tractus solitarius
- PVN
hypothalamic paraventricular nucleus
- RVLM
rostral ventrolateral medullary pressor area
- SEM
standard error of the means
- TBS
Tris buffered saline
- VGLUT2
vesicular glutamate transporter-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Accorsi-Mendonca D, Leao RM, Aguiar JF, Varanda WA, Machado BH. Urethane inhibits the GABAergic neurotransmission in the nucleus of the solitary tract of rat brain stem slices. Am J Physiol Regul Integr Comp Physiol. 2007;292:R396–R402. doi: 10.1152/ajpregu.00776.2005. [DOI] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Jin YH, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci. 2006;26:6131–6142. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks D, Harris MC. Activation within dorsal medullary nuclei following stimulation in the hypothalamic paraventricular nucleus in rats. Pflugers Arch. 1987;408:619–627. doi: 10.1007/BF00581165. [DOI] [PubMed] [Google Scholar]
- Braga DC, Mori E, Higa KT, Morris M, Michelini LC. Central oxytocin modulates exercise-induced tachycardia. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1474–R1482. doi: 10.1152/ajpregu.2000.278.6.R1474. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Coote JH. The hypothalamus and cardiovascular regulation. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural mechanisms of cardiovascular regulation. Boston, MA, USA: Kluwer Academic Publishers; 2004. pp. 117–146. [Google Scholar]
- Darlington DN, Miyamoto M, Keil LC, Dallman MF. Paraventricular stimulation with glutamate elicits bradycardia and pituitary responses. Am J Physiol Regul Integr Comp Physiol. 1989;256:R112–R119. doi: 10.1152/ajpregu.1989.256.1.R112. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Bhatnagar T, Sapru HN. Cardiovascular responses to microinjections of glutamate into the nTS of unanesthetized supracollicular decerebrate rats. Brain Res. 1998;801:88–100. doi: 10.1016/s0006-8993(98)00550-2. [DOI] [PubMed] [Google Scholar]
- Duan YF, Kopin IJ, Goldstein DS. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 1999;277:R403–R411. doi: 10.1152/ajpregu.1999.277.2.R403. [DOI] [PubMed] [Google Scholar]
- Dufloth DL, Morris M, Michelini LC. Modulation of exercise tachycardia by vasopressin in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1271–R1282. doi: 10.1152/ajpregu.1997.273.4.R1271. [DOI] [PubMed] [Google Scholar]
- Faber JE. Effects of althesin and urethane-chloralose on neurohumoral cardiovascular regulation. Am J Physiol Regul Integr Comp Physiol. 1989;256:R757–R765. doi: 10.1152/ajpregu.1989.256.3.R757. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: Glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hardy SGP. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/s0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, EL Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21(RC181):1–6. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am J Physiol Regul Integr Comp Physiol. 2002;282:R537–R545. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- Kannan H, Yamashita H. Electrophysiological study of paraventricular nucleus neurons projecting to the dorsomedial medulla and their response to baroreceptor stimulation in rats. Brain Res. 1983;279:31–40. doi: 10.1016/0006-8993(83)90160-9. [DOI] [PubMed] [Google Scholar]
- Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res. 1985;329:205–212. doi: 10.1016/0006-8993(85)90526-8. [DOI] [PubMed] [Google Scholar]
- Kasamatsu K, Chitravanshi VC, Sapru HN. Depressor and bradycardic responses to microinjections of endomorphin-2 into the NTS are mediated via ionotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 2004;287:R715–R728. doi: 10.1152/ajpregu.00642.2003. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Oomura Y, Kurosawa M. Effects of chemical stimulation of paraventricular nucleus on adrenal and renal nerve activity in rats. Neurosci Lett. 1988;86:195–200. doi: 10.1016/0304-3940(88)90570-8. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res. 2006;1102:117–126. doi: 10.1016/j.brainres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- Matsuguchi H, Sharabi FM, Gordon FJ, Johnson AK, Schmid PG. Blood pressure and heart rate responses to microinjection of vasopressin into the nucleus tractus solitarius region of the rat. Neuropharmacology. 1982;21:687–693. doi: 10.1016/0028-3908(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Michelini LC. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: Reflex mechanisms and changes during exercise. Clin Exp Pharmacol Physiol. 2007;34:369–376. doi: 10.1111/j.1440-1681.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- Osborn JW, England SK. Normalization of arterial pressure after barodenervation: role of pressure natriuresis. Am J Physiol Regul Integr Comp Physiol. 1990;259:R1172–R1180. doi: 10.1152/ajpregu.1990.259.6.R1172. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Geoffrey Harris Memorial Lecture, Kitakyushu, Japan, October1998. Front Neuroendocrinol. 1999;20:270–295. doi: 10.1006/frne.1999.0186. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Rivest S. Does circulating leptin have the ability to cross the blood-brain barrier and target neurons directly? Endocrinology. 2002;143:3211–3213. doi: 10.1210/en.2002-220655. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Gastric-vagal solitary neurons excited by paraventricular nucleus microstimulation. J Auton Nerv Syst. 1985;14:351–362. doi: 10.1016/0165-1838(85)90081-5. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol. 2002;29:491–496. doi: 10.1046/j.1440-1681.2002.03661.x. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Neurotransmitters in the nucleus tractus solitarius mediating cardiovascular function. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural mechanisms of cardiovascular regulation. Boston, MA, USA: Kluwer Academic Publishers; 2004. pp. 81–98. [Google Scholar]
- Sapru HN, Gonzalez ER, Krieger AJ. Aortic nerve stimulation in the rat: cardiovascular and respiratory responses. Brain Res Bull. 1981;6:393–398. doi: 10.1016/s0361-9230(81)80009-3. [DOI] [PubMed] [Google Scholar]
- Shen E, Dun SL, Ren C, Dun NJ. Hypovolemia induces Fos-like immunoreactivity in neurons of the rat supraoptic and paraventricular nuclei. J Auton Nerv Syst. 1992;37:227–230. doi: 10.1016/0165-1838(92)90045-i. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Zhang J, Toney GM, Mifflin SW. Temporal processing of aortic nerve evoked activity in the nucleus of the solitary tract. J Neurophysiol. 1996;76:3750–3757. doi: 10.1152/jn.1996.76.6.3750. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Schrell U. Evidence for direct projection from oxytocin and vasopressin neurons in the hypothalamic paraventricular nucleus to the medulla oblongata: immunohistochemical visualization of both the horseradish peroxidase transported and the peptide produced by the same neurons. Neurosci Lett. 1981;22:211–217. [Google Scholar]
- Stern JE. Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol. 2004;84:197–215. doi: 10.1016/j.pbiomolbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Talman WT, Granata AR, Reis DJ. Glutamatergic mechanisms in the nucleus tractus solitarius in blood pressure control. Fed Proc. 1984;43:39–44. [PubMed] [Google Scholar]
- Viard E, Sapru HN. Cardiovascular responses to activation of metabotropic glutamate receptors in the nTS of the rat. Brain Res. 2002;952:308–321. doi: 10.1016/s0006-8993(02)03260-2. [DOI] [PubMed] [Google Scholar]
- Willette RN, Krieger AJ, Barcas PP, Sapru HN. Medullary GABA receptors and the regulation of blood pressure in the rat. J Pharmacol Exp Ther. 1983;226:893–899. [PubMed] [Google Scholar]
- Yamashita H, Kannan H, Kasai M, Osaka T. Decrease in blood pressure by stimulation of the rat hypothalamic paraventricular nucleus with L-glutamate or weak current. J Auton Nerv Syst. 1987;19:229–234. doi: 10.1016/0165-1838(87)90069-5. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol. 1998;511:733–745. doi: 10.1111/j.1469-7793.1998.733bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]