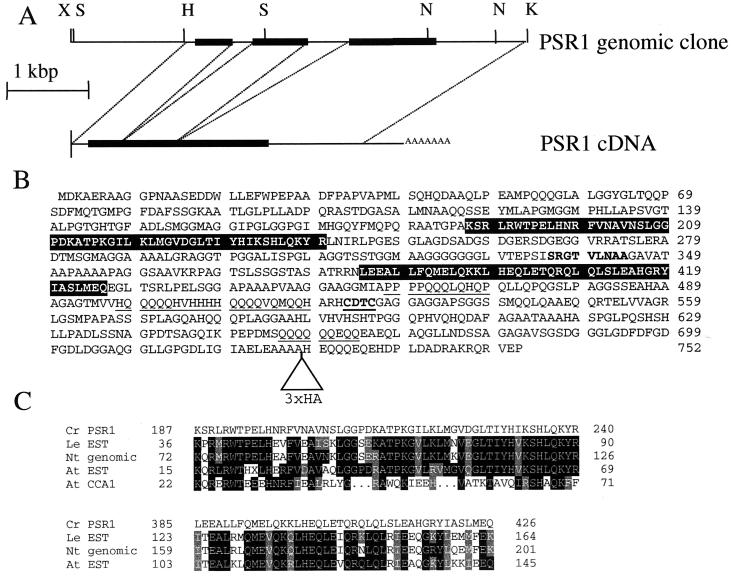
Figure 2.
Psr1 gene structure and sequence. (A) Structure of Psr1 cDNA and genomic clones. Abbreviations for restriction enzyme sites are X, XbaI; H, HindIII; S, SalI; N, NotI; and K, KpnI. The solid rectangle indicates the position of the ORF, and dashed lines indicate the sites of intron splicing. (B) Predicted amino acid sequence of Psr1. Amino acid sequence begins with the first in-frame methionine after a stop codon in the cDNA. Features of Psr1 include: a helix–turn–helix dimerization domain (bold), a putative metal binding site (bold, underlined), three glutamine-rich regions that share similarity to activation domains of homeobox proteins in various organisms (underlined; ref. 19), and two regions that are similar to deduced sequences in vascular plant proteins (highlighted in a black background). The triangle near the C terminus indicates an in-frame insertion of the 3×HA (HA epitope) tag. (C) Alignment of predicted Psr1 and vascular plant amino acid sequences. The two domains of the predicted protein sequence of Psr1 (amino acids 187–240 and 386–428) are aligned with proteins deduced from cDNA sequences of Lycopersicon esculentum (Le EST, accession no. AI485303), A. thaliana (At EST, combination of accession nos. H76190, T45758, T04440, T76484, and T41999), a genomic clone of Nicotiana tabacum (NT genomic, accession no. AB017693), and the distantly related myb DNA-binding domain of Cca1 (only in the upper alignment, At CCA1, accession no. U28422). Identical residues are in black and conserved residues are in gray.