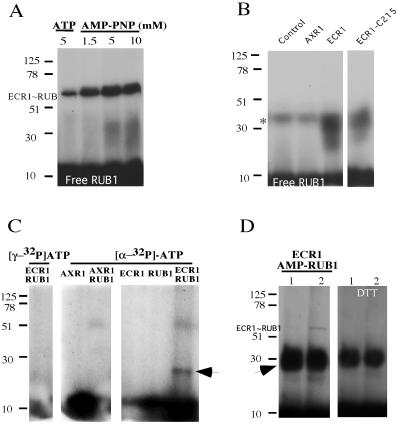
Figure 1.
RUB1-AMP is an intermediate product of RUB1 activation. (A) Thiolester reactions containing radiolabeled RUB1 and 5 mM ATP or different AMP-PNP concentrations were performed. These reactions contained protein extract prepared from bacteria expressing recombinant H6-AXR1 and H6-ECR1 (see Experimental Procedures). The reaction products were separated by SDS/PAGE in the absence of DTT. (B) Thiolester assay of ECR1 or AXR1 subunits. Radiolabeled RUB1 was used in thiolester reactions that contained 10 mM ATP and bacterial protein extract containing recombinant H6-AXR1, H6-ECR1, or H6-ECR1215A proteins. The reactions were stopped with 4× SDS/DTT loading buffer. The DTT-resistant band at 25–35 kDa was formed when the reaction was performed with ECR1 or ECR1215A. Asterisks indicate residual GST-32P-RUB1 remaining after thrombin digestion. (C) Purified H6-AXR1 or ECR1 were incubated with [α-32P]ATP or [γ-32P]ATP and cold RUB1 protein. A DTT-resistant product at ≈25 kDa (arrow) was generated only when ECR1 was incubated with [α-32P]ATP. The label at the bottom of the gel is unincorporated ATP. (D) AMP-32P-RUB1 (arrow) was generated by incubation of ECR1 with 32P-RUB1 and ATP. After removing the ATP by ammonium sulfate precipitation, either buffer (lane 1) or H6-AXR1 (lane 2) was added to the reactions.