Abstract
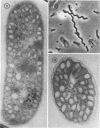
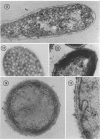
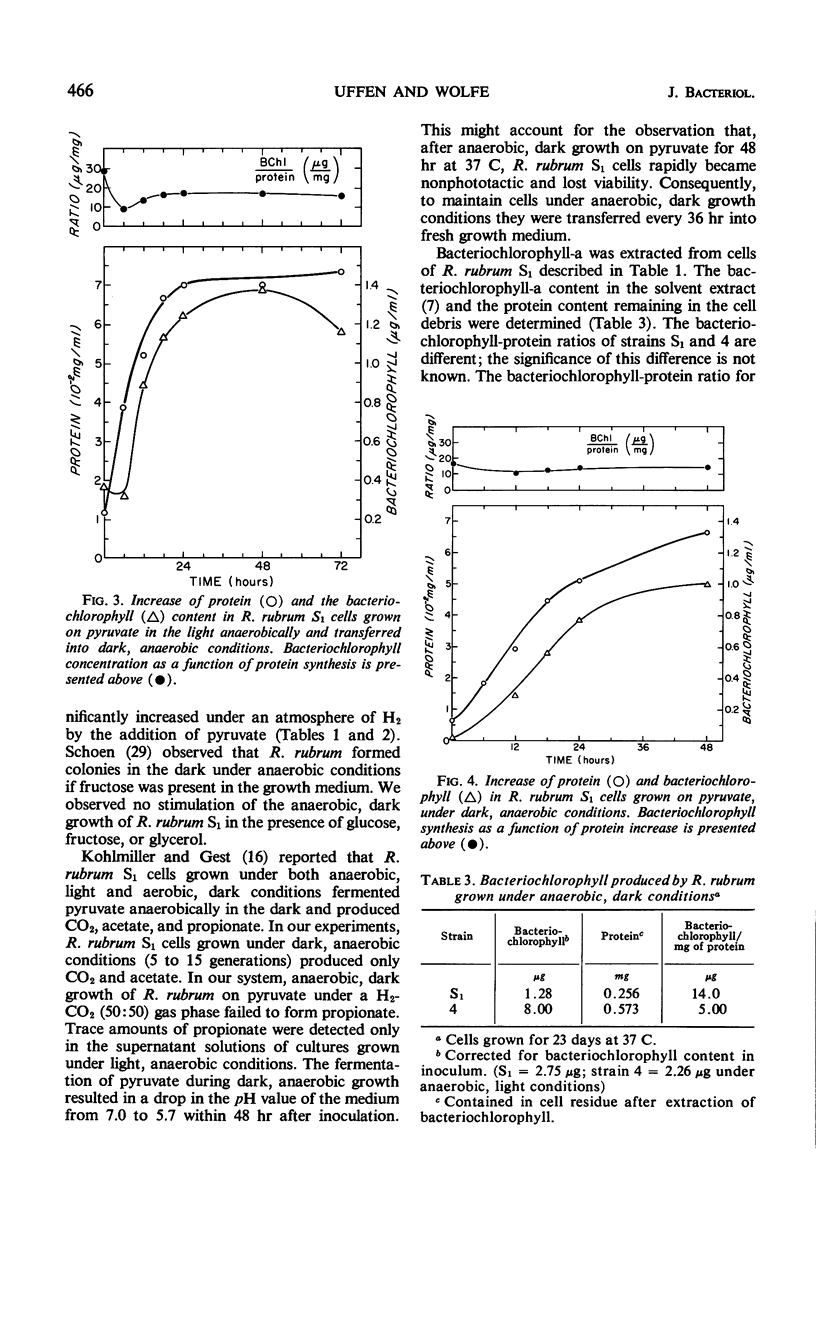
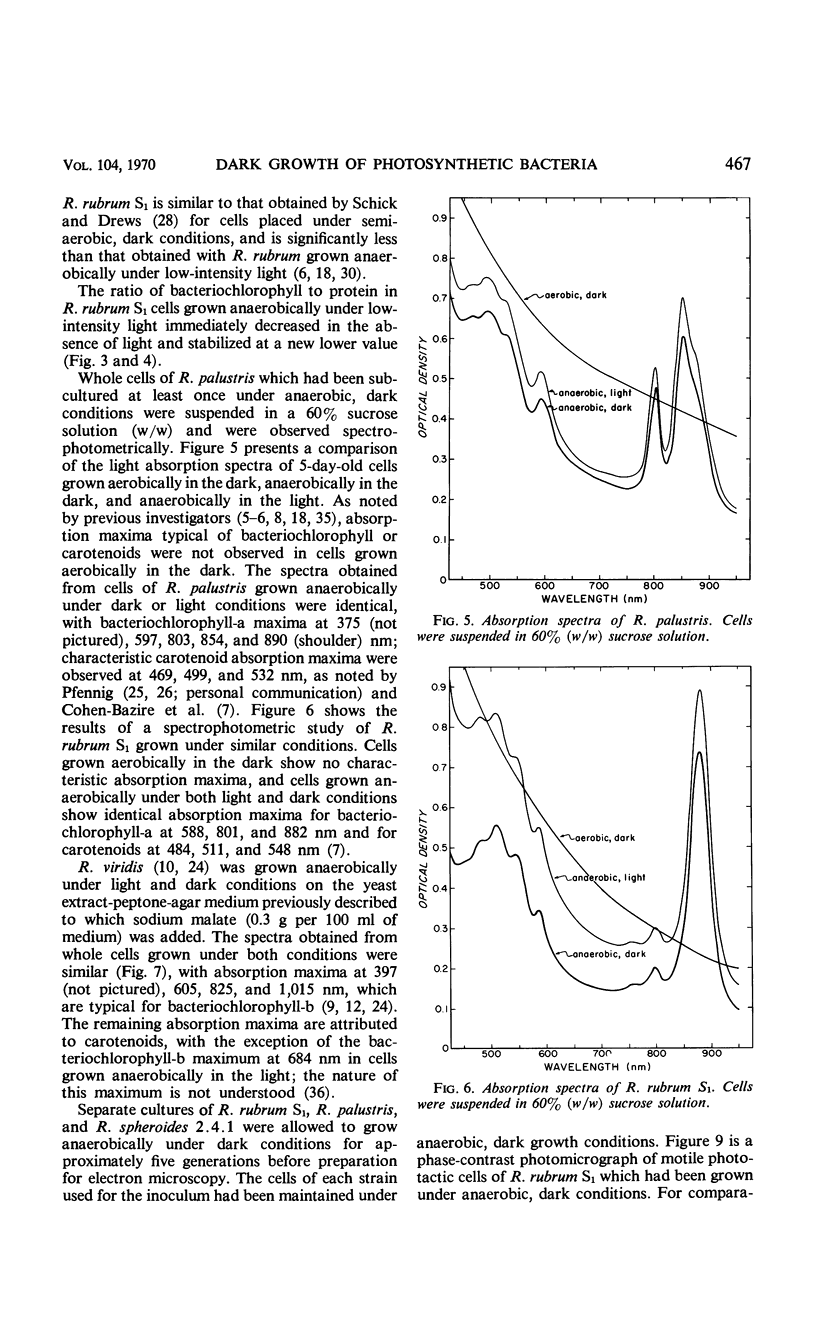
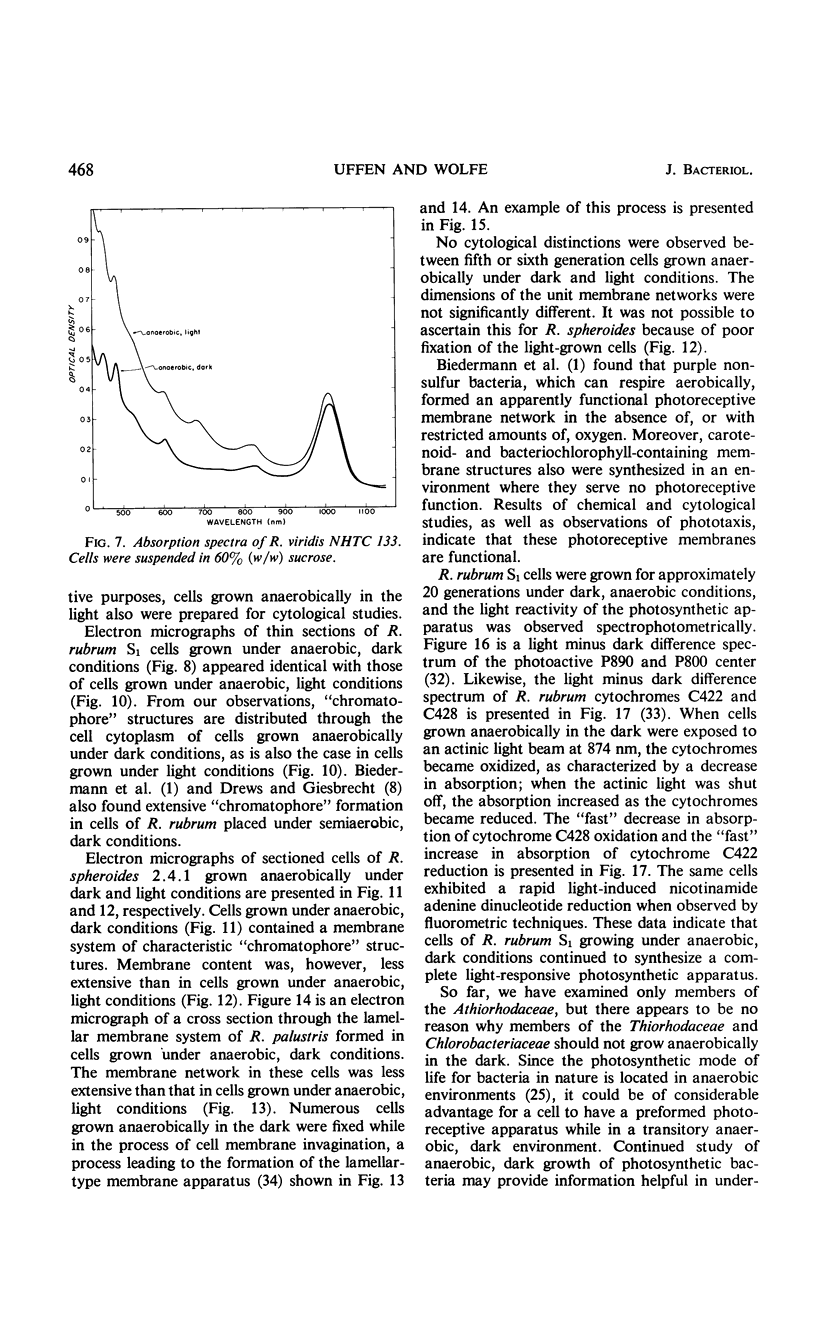
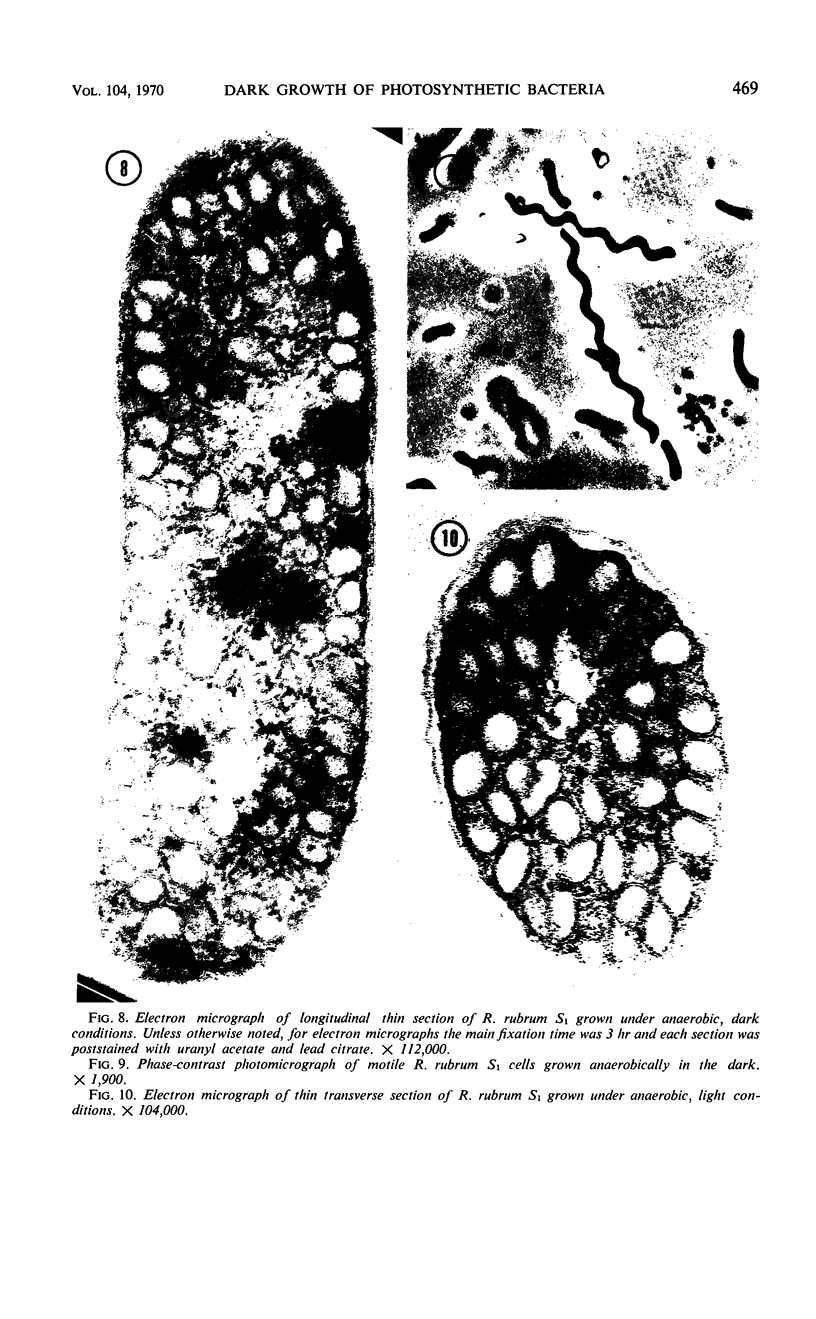
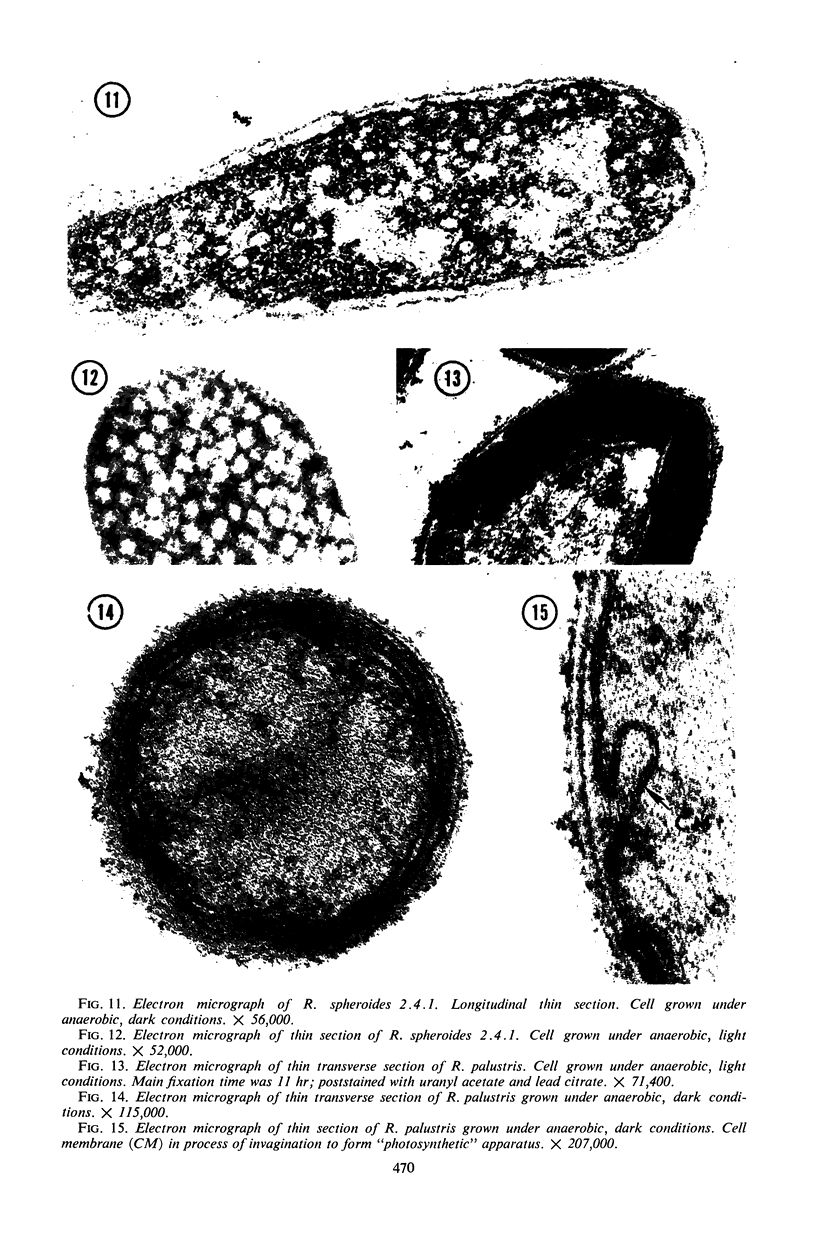
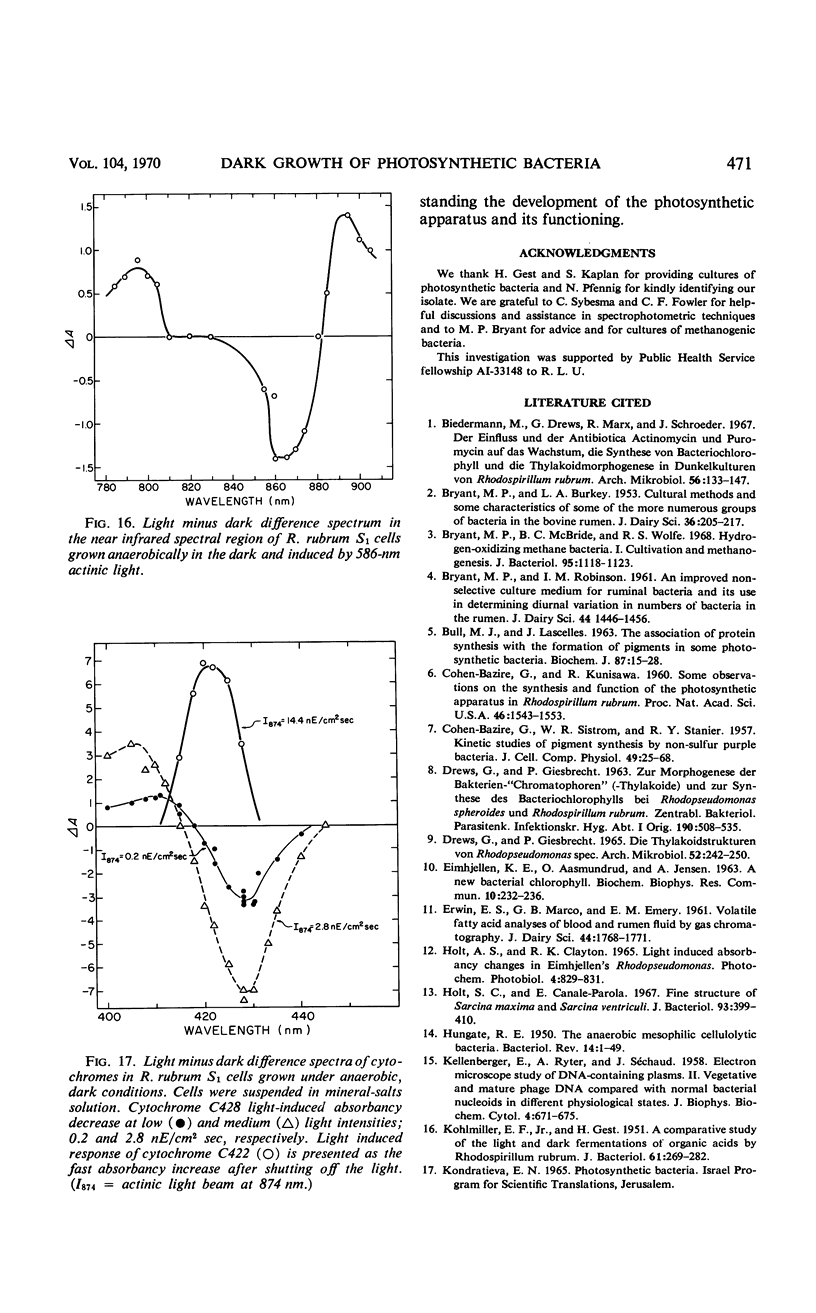
Purple nonsulfur photosynthetic bacteria were cultured anaerobically in the absence of light by a modification of the Hungate technique. Growth was slow and resembled that of fastidious anaerobes; on yeast extract-peptone-agar medium, each cell produced about 16 descendants in 15 to 20 days. Growth was stimulated by addition of ethyl alcohol, acetate and H2, or pyruvate and H2. Cells grown in the presence of pyruvate and H2 produced acetate and CO2; each cell produced approximately 10 descendants in 24 hr under anaerobic, dark conditions. Spectrophotometric evidence obtained from cells which were the product of five generations suggests no difference between the bacteriochlorophyll and carotenoids synthesized by cells grown anaerobically under dark or light conditions. Likewise, the ultrastructure of the photosynthetic apparatus in cells grown anaerobically in the dark and in the light appears similar.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULL M. J., LASCELLES J. The association of protein synthesis with formation of pigments in some photosynthetic bacteria. Biochem J. 1963 Apr;87:15–28. doi: 10.1042/bj0870015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann M., Drews G., Marx R., Schröder J. Der Einfluss des Sauerstoffpartialdruckes und der Antibiotica Actinomycin und Puromycin auf das Wachstum, die Synthese von Bacteriochlorophyll und die Thylakoidmorphogenese in Dunkelkulturen von Rhodospirillum rubrum. Arch Mikrobiol. 1967 Feb 20;56(2):133–147. [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R. SOME OBSERVATIONS ON THE SYNTHESIS AND FUNCTION OF THE PHOTOSYNTHETIC APPARATUS IN RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1543–1553. doi: 10.1073/pnas.46.12.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREWS G., GIESBRECHT P. ZUR MORPHOGENESE DER BAKTERIEN-"CHROMATOPHOREN" (-THYLAKOIDE) UND ZUR SYNTHESE DES BACTERIOCHLOROPHYLLS BEI RHODOPSEUDOMANAS SPHEROIDES UND RHODOSPIRILLUM RUBURM. Zentralbl Bakteriol Orig. 1963 Dec;190:508–535. [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt A. S., Clayton R. K. Light-induced absorbancy changes in Eimhjellen's Rhodopseudomonas. Photochem Photobiol. 1965 Sep;4(4):829–831. doi: 10.1111/j.1751-1097.1965.tb07925.x. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Canale-Parola E. Fine structure of Sarcina maxima and Sarcina ventriculi. J Bacteriol. 1967 Jan;93(1):399–410. doi: 10.1128/jb.93.1.399-410.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLMILLER E. F., Jr, GEST H. A comparative study of the light and dark fermentations of organic acids by Rhodo-spirillum rubrum. J Bacteriol. 1951 Mar;61(3):269–282. doi: 10.1128/jb.61.3.269-282.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. M., Nadler K. D. Energy transfer and cytochrome function in a new type of photosynthetic bacterium. Photochem Photobiol. 1965 Sep;4(4):783–791. doi: 10.1111/j.1751-1097.1965.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Rhodopseudomonas acidophila, sp. n., a new species of the budding purple nonsulfur bacteria. J Bacteriol. 1969 Aug;99(2):597–602. doi: 10.1128/jb.99.2.597-602.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick J., Drews G. The morphogenesis of the bacterial photosynthetic apparatus. 3. The features of a pheophytin-protein-carbohydrate complex excreted by the mutant M 46 of Rodospirillum rubrum. Biochim Biophys Acta. 1969 Jun 3;183(1):215–229. doi: 10.1016/0005-2736(69)90145-x. [DOI] [PubMed] [Google Scholar]
- Schön G. Fructoserverwertung und Bacteriochlorophyllsynthese in anaeroben Dunkel- und Lichtkulturen von Rhodospirillum rubrum. Arch Mikrobiol. 1968;63(4):362–375. [PubMed] [Google Scholar]
- Sybesma C., Fowler C. F. Evidence for two light-driven reactions in the purple photosynthetic bacterium, Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1343–1348. doi: 10.1073/pnas.61.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybesma C. Light-induced reactions of P890 and P800 in the purple photosynthetic bacterium Rhodospirillum rubrum. Biochim Biophys Acta. 1969 Jan 14;172(1):177–179. doi: 10.1016/0005-2728(69)90104-2. [DOI] [PubMed] [Google Scholar]
- Tauschel H. D., Drews G. Thylakoidmorphogenese bei Rhodopseudomonas palustirs. Arch Mikrobiol. 1967;59(4):381–404. [PubMed] [Google Scholar]
- Thore A., Keister D. L., San Pietro A. Studies on the respiratory system of aerobically (dark) and anaerobically (light) grown Rhodospirillum rubrum. Arch Mikrobiol. 1969;67(4):378–396. doi: 10.1007/BF00412584. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Olson J. M., Williams D. M., Clayton M. L. Isolation of the reaction center of Rhodopseudomonas viridis. Biochim Biophys Acta. 1969 Feb 25;172(2):351–354. doi: 10.1016/0005-2728(69)90083-8. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Hydrogenase measurement with photochemically reduced methyl viologen. J Bacteriol. 1969 Apr;98(1):51–55. doi: 10.1128/jb.98.1.51-55.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]