Abstract
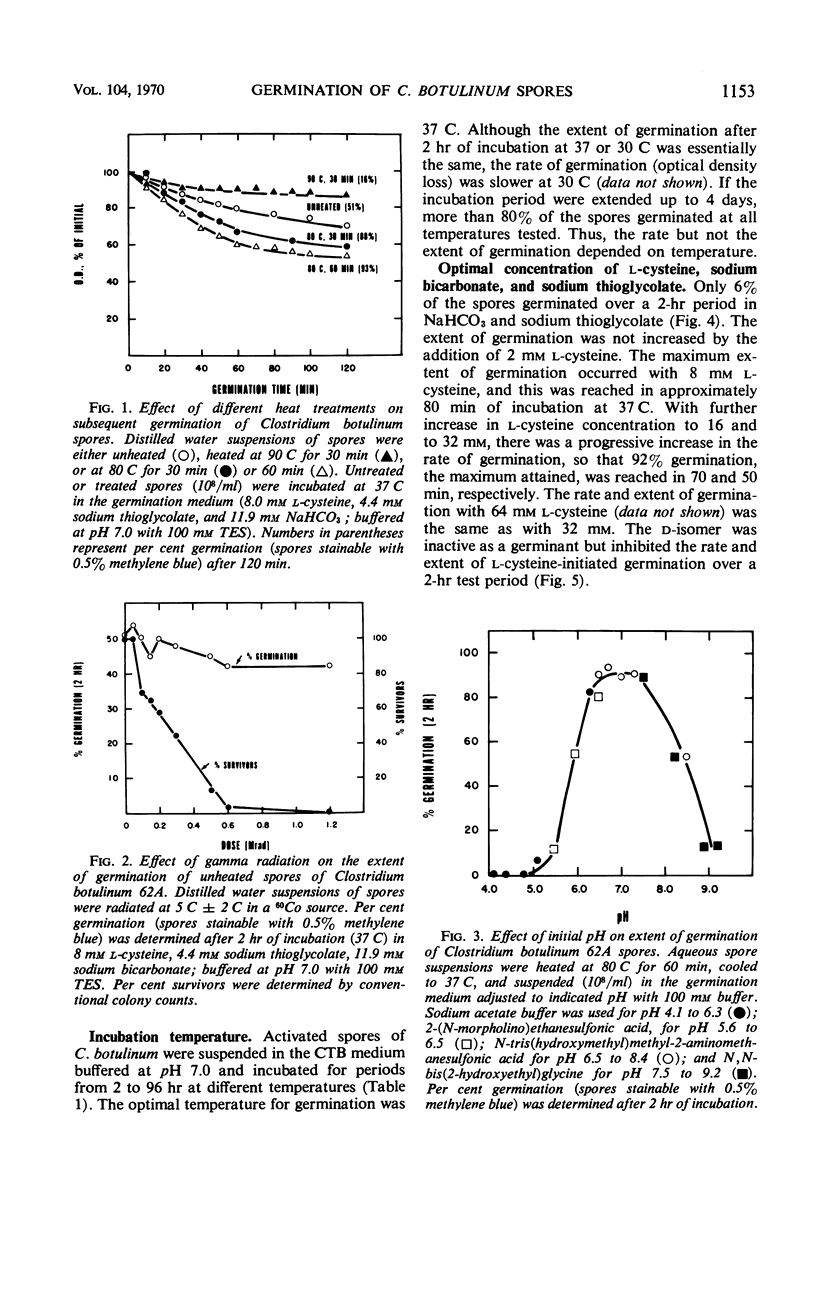
Spores of Clostridium botulinum type 62A were germinated in a chemically defined medium (8 mm l-cysteine, 11.9 mm sodium bicarbonate, 4.4 mm sodium thioglycolate; buffered with 100 mm TES, pH 7.0). The rate and extent of germination were increased when an aqueous spore suspension was heated sublethally (80 C, 60 min) before addition to the germination medium. Neither sublethal nor lethal doses of gamma radiation had any marked effect on subsequent germination. Maximum germination (>90% in 2 hr) in the defined medium occurred in the pH range of 6.5 to 7.5, at 30 to 37 C, with an l-cysteine level of 8 mm. Increasing l-cysteine to 32 mm increased the rate (over that with 8 mm l-cysteine) but not the extent of germination. The rate and extent of germination increased with NaHCO3 addition to 8.3 mm, but increasing levels to 11.9 mm had no further effect. For maximum germination, 2.2 mm sodium thioglycolate was required and higher levels (to 8.8 mm) had no further enhancing or inhibitory effect. Under optimal conditions for germination, 97% of the spores had become heat sensitive; 98% had become sensitive to radiation; 88 and 91% had become phase dark and stainable, respectively, and the spore suspension had lost 46% of its initial optical density by 2 hr. Loss of heat resistance preceded loss of radiation resistance, acquisition of stainability, and phase darkening by about 12 min.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anellis A., Berkowitz D., Jarboe C., el Bisi H. M. Radiation sterilization of prototype military foods. II. Cured ham. Appl Microbiol. 1967 Jan;15(1):166–177. doi: 10.1128/am.15.1.166-177.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTILOW R. N. Fermentative activities of control and radiation-"killed" spores of Clostridium botulinum. J Bacteriol. 1962 Dec;84:1268–1273. doi: 10.1128/jb.84.6.1268-1273.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran H. R., Evans F. R. Heat Activation Inducing Germination in the Spores of Thermotolerant and Thermophilic Aerobic Bacteria. J Bacteriol. 1945 Apr;49(4):335–346. doi: 10.1128/jb.49.4.335-346.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E., Goodnow R., Grecz N. Changes in resistance to radiation and heat during sporulation and germination of Clostridium botulinum 33A. J Bacteriol. 1970 May;102(2):590–592. doi: 10.1128/jb.102.2.590-592.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS P. A. FACTORS AFFECTING THE GERMINATION OF SPORES OF CLOSTRIDIUM BIFERMENTANS. J Gen Microbiol. 1964 Oct;37:41–48. doi: 10.1099/00221287-37-1-41. [DOI] [PubMed] [Google Scholar]
- GRECZ N., ANELLIS A., SCHNEIDER M. D. Procedure for cleaning of Clostridium botulinum spores. J Bacteriol. 1962 Sep;84:552–558. doi: 10.1128/jb.84.3.552-558.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P. A. The activation of spores of Clostridium bifermentans. J Gen Microbiol. 1967 Feb;46(2):285–291. doi: 10.1099/00221287-46-2-285. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Ordal Z. J. Activation of spores of Bacillus cereus by gamma-radiation. J Gen Microbiol. 1968 Jan;50(1):77–84. doi: 10.1099/00221287-50-1-77. [DOI] [PubMed] [Google Scholar]
- Grecz N., Lin C. A., Tang T., So W. L., Sehgal L. R. The nature of heat resistant toxin in spores of Clostridium botulinum. Jpn J Microbiol. 1967 Dec;11(4):384–394. doi: 10.1111/j.1348-0421.1967.tb00362.x. [DOI] [PubMed] [Google Scholar]
- HITZMAN D. O., HALVORSON H. O., UKITA T. Requirements for production and germination of spores of anaerobic bacteria. J Bacteriol. 1957 Jul;74(1):1–7. doi: 10.1002/path.1700740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt M. T., Holmes P. K., Levinson H. S. Activation of Bacillus megaterium spore germination by water in the vapor phase. Biochem Biophys Res Commun. 1966 Sep 8;24(5):701–704. doi: 10.1016/0006-291x(66)90381-0. [DOI] [PubMed] [Google Scholar]
- KEYNAN A., EVANCHIK Z., HALVORSON H. O., HASTINGS J. W. ACTIVATION OF BACTERIAL ENDOSPORES. J Bacteriol. 1964 Aug;88:313–318. doi: 10.1128/jb.88.2.313-318.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. Some effects of heat and ionizing radiation on spores of Bacillus megaterium. J Bacteriol. 1960 Oct;80:441–451. doi: 10.1128/jb.80.4.441-451.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T. Sequence of events during Bacillus megaterim spore germination. J Bacteriol. 1966 May;91(5):1811–1818. doi: 10.1128/jb.91.5.1811-1818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREADWELL P. E., JANN G. J., SALLE A. J. Studies on factors affecting the rapid germination of spores of Clostridium botulinum. J Bacteriol. 1958 Nov;76(5):549–556. doi: 10.1128/jb.76.5.549-556.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara M., Frank H. A. Sequence of events during germination of putrefactive anaerobe 3679 spores. J Bacteriol. 1967 Sep;94(3):506–511. doi: 10.1128/jb.94.3.506-511.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLEY B. C., COLLIER R. E. CHANGES IN THERMORESISTANCE OF CLOSTRIDIUM ROSEUM AS RELATED TO THE INTRACELLULAR CONTENT OF CALCIUM AND DIPICOLINIC ACID. Can J Microbiol. 1965 Apr;11:279–285. doi: 10.1139/m65-034. [DOI] [PubMed] [Google Scholar]
- Williams-Walls N. J. Effects on growth and toxin production of exposure of spores of Clostridium botulinum type F to sublethal doses of gamma irradiation. Appl Microbiol. 1969 Jan;17(1):128–134. doi: 10.1128/am.17.1.128-134.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


