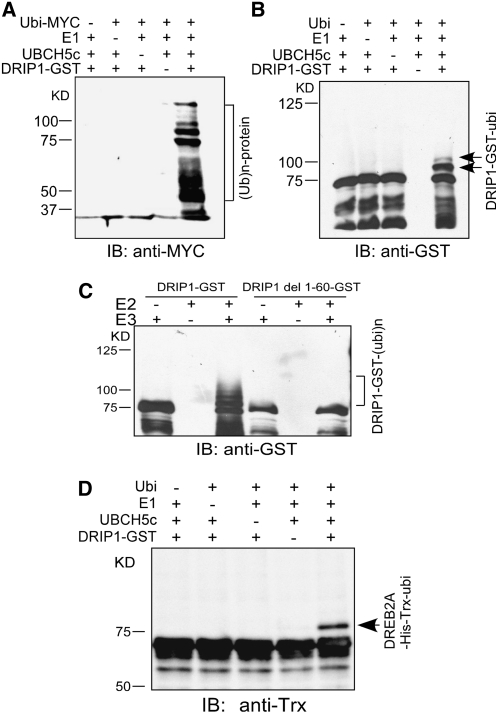
Figure 3.
DRIP1 Functions as an E3 Ubiquitin Ligase and Mediates DREB2A Protein Ubiquitination.
(A) In the presence of the ubiquitin-myc, E1, and E2 enzymes, DRIP1-GST fusion proteins display ubiquitin E3 ligase activity. Protein bands with ubiquitin attached were detected by anti-myc immunoblot (IB) analysis (10% SDS-PAGE).
(B) Detection of DRIP1-GST autoubiquitination. DRIP1-GST fusion proteins were detected with a GST antibody, and shifted bands indicate the attachment of one or two ubiquitin molecules (6% SDS-PAGE).
(C) Assessment of E3 ubiquitin ligase activity. Wild-type and RING domain–deleted DRIP1-GST fusion proteins (DRIP1 del1-60-GST) were tested for E3 ubiquitin ligase activity in the presence of UBCH5c (E2), E1, and ubiquitin. A GST antibody was used to detect DRIP1-GST fusion protein (10% SDS-PAGE).
(D) DRIP1 mediates the ubiquitination of DREB2A protein. The full-length DREB2A protein was fused with His and Trx tags (DREB2A-His-Trx) and used as the substrate for the assay. Anti-Trx was used in the immunoblot analysis for the detection of Trx-tagged substrate protein (6% SDS-PAGE).