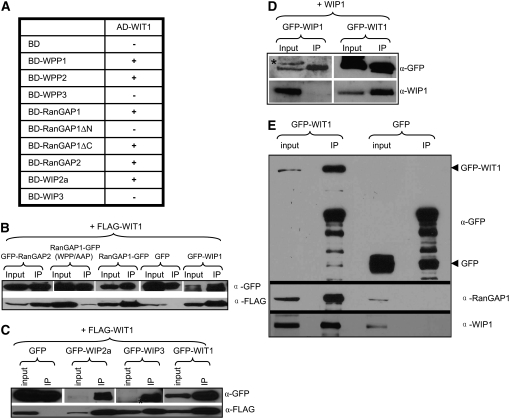
Figure 2.
WIT1 Interacts with WPP1, WPP2, RanGAP, and WIP Family Members and with Itself.
(A) Interaction of WIT1 with WIP family or WPP domain proteins in yeast two-hybrid assays. RanGAP1ΔC, WPP domain of RanGAP1; RanGAP1ΔN, RanGAP1 without WPP domain; AD, GAL4 activation domain; BD, GAL4 DNA binding domain. Plus (+), positive interaction; minus (−), no interaction. Both WIP1 and WIT1 self-activate as BD fusions, which were therefore not included in this assay.
(B) WIT1 interacts with RanGAP1 and 2 and with WIP1 in planta. Interaction with RanGAP1 is abolished by the WPP/AAP mutation in the WPP domain of RanGAP1. FLAG-WIT1 was coexpressed with GFP, RanGAP1-GFP, GFP-RanGAP2, GFP-WIP1, or RanGAP1 (WPP/AAP)-GFP in N. benthamiana. Immunoprecipitation was performed using the anti-GFP antibody, and coimmunoprecipitated protein was detected with the anti-FLAG antibody.
(C) WIT1 interacts with WIP proteins and has homodimerization ability in planta. FLAG-WIT1 was coexpressed with GFP, GFP-WIP2a, GFP-WIP3, or GFP-WIT1 in N. benthamiana. Immunoprecipitation was performed using the anti-GFP antibody, and coimmunoprecipitated protein was detected with the anti-FLAG antibody. A nonspecific band detected with the anti-GFP antibody is indicated with an asterisk.
(D) WIP1 does not homodimerize in planta. WIP1 was coexpressed with GFP-WIP1 or GFP-WIT1 constructs in N. benthamiana. Immunoprecipitation was performed using the anti-GFP antibody, and coimmunoprecipitated protein was detected with the anti-WIP1 antibody. A nonspecific band detected with the anti-GFP antibody is indicated with an asterisk.
(E) WIT1 interacts with endogenous RanGAP1 and WIP1 in Arabidopsis. Samples immunoprecipitated from GFP and GFP-WIT1 transgenic lines using a monoclonal anti-GFP antibody were probed with the anti-RanGAP1 and the anti-WIP1 antibody.