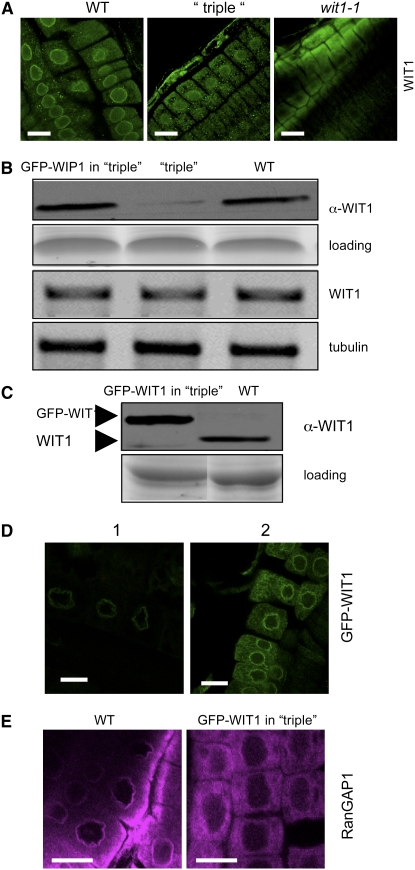
Figure 5.
WIT1 Cannot Functionally Replace the WIP Family.
(A) Immunofluorescence localization of WIT1 in the wild-type, the wip1-1 wip2-1 wip3-1 triple mutant (“triple”), and the wit1-1 mutant. Bars = 10 μm.
(B) WIT1 protein level is reduced in the wip1-1 wip2-1 wip3-1 triple mutant (“triple”). Protein extracts from the wild type, the wip1-1 wip2-1 wip3-1 triple mutant, and GFP-WIP1 in the wip1-1 wip2-1 wip3-1 triple mutant were probed with anti-WIT1 antibody. A section of a Coomassie blue–stained replica gel is shown as loading control. WIT1 mRNA level is not changed in the wip1-1 wip2-1 wip3-1 triple mutant. The third panel from the top shows RT-PCR analysis with WIT1-specific primers. The bottom panel shows tubulin as RT-PCR control.
(C) Protein extract from the wild type and GFP-WIT1 in the wip1-1 wip2-1 wip3-1 triple mutant (“triple”) were probed with anti-WIT1 antibody. A Coomassie blue–stained replica gel shown at the bottom represents loading control.
(D) Overexpressed GFP-WIT1 is targeted to the NE in the wip1-1 wip2-1 wip3-1 triple mutant. GFP-WIT1 in the wip1-1 wip2-1 wip3-1 triple mutant was detected by live confocal imaging (1) or by immunofluorescence with a polyclonal anti-GFP antibody (ab290) (2). Bars = 10 μm.
(E) Immunofluorescence localization of RanGAP1 in root tip cells in the wild-type background (left panel) and in wip1-1 wip2-1 wip3-1/GFP-WIT1 (right panel). Bars = 10 μm.