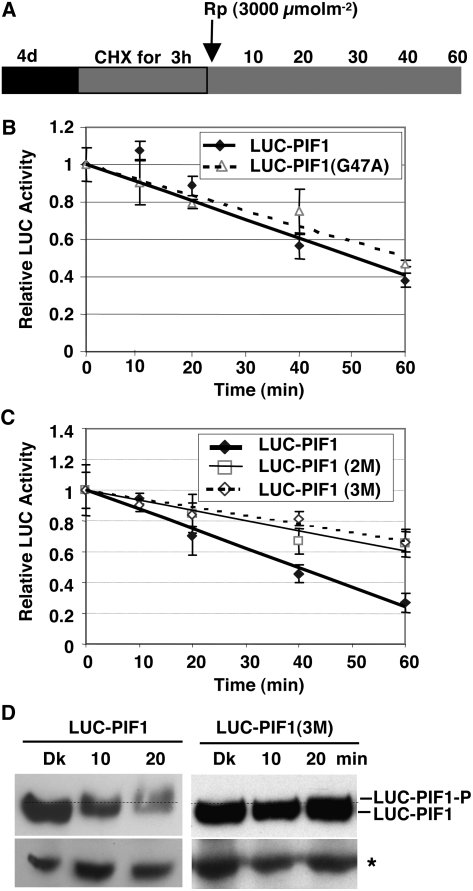
Figure 6.
Interactions with the Pfr Form of phyA and phyB Are Necessary for the Light-Induced Phosphorylation and Degradation of PIF1.
(A) Design of the cycloheximide chase assays. Relative lucerifase activity for phy interaction–deficient mutants was measured in 4-d-old dark-grown seedlings pretreated with cycloheximide (CHX) in the dark for 3 h, exposed to R (3000 μmol·m−2) light, and then incubated in the dark for the indicated times (min).
(B) and (C) Assays show the kinetics of degradation of LUC-PIF1-G47A (B) and LUC-PIF1-2M and LUC-PIF1-3M (C) compared with wild-type LUC-PIF1. LUC-PIF1G47A is deficient in phyB interaction, LUC-PIF1-2M is deficient in phyA interaction, and LUC-PIF1-3M is deficient in both phyA and phyB interaction, as shown in Figure 5. Means ± se of five biological replicates are shown.
(D) The abundance and phosphorylation status of LUC-PIF1 and LUC-PIF1-3M fusion proteins prior to and after exposure to Rp determined on protein gel blots using anti-LUC antibody. The dotted line separates the two forms of PIF1. The asterisk denotes a cross-reacting band.