Abstract
Background and purpose:
Oncocalyxone A (OncoA) has a concentration-dependent anti-platelet activity. The present study aimed to further understand the mechanisms related to this effect.
Experimental approach:
Human platelet aggregation was measured by means of a turbidimetric method. OncoA (32–256 μM) was tested against several platelet-aggregating agents, such as adenosine diphosphate (ADP), collagen, arachidonic acid (AA), ristocetin and thrombin.
Key results:
OncoA completely inhibited platelet aggregation with a calculated mean inhibitory concentration (IC50-μM) of 122 for ADP, 161 for collagen, 159 for AA, 169 for ristocetin and 85 for thrombin. The anti-aggregatory activity of OncoA was not inhibited by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). OncoA, at a concentration that caused no significant anti-aggregatory activity, potentiated sodium nitroprusside (SNP) anti-aggregatory activity (18.8±2.9%-SNP vs 85.0±8.2%-SNP+OncoA). The levels of nitric oxide (NO) or cAMP were not altered by OncoA while cGMP levels were increased more than 10-fold by OncoA in resting or ADP-activated platelets. Flow cytometry revealed that OncoA does not interact with receptors for fibrinogen, collagen or P-selectin. Nevertheless, OncoA decreased the binding of antibodies to GP Ibα, a glycoprotein that is related both to von Willebrand factor and to thrombin-induced platelet aggregation.
Conclusion and implications:
OncoA showed anti-aggregatory activity in platelets that was associated with increased cGMP levels, not dependent on NO and with blocking GP Ibα glycoprotein. This new mechanism has the prospect of leading to new anti-thrombotic drugs.
Keywords: quinones, Auxemma oncocalyx, oncocalyxone, antiplatelet activity, human platelets
Introduction
Auxemma oncocalyx Taub. (Boraginaceae) known as ‘pau-branco' is a tree native to the Northeast of Brazil. Pharmacological studies using a water-soluble fraction of an ethanolic extract obtained from the heartwood of A. oncocalyx was previously shown to inhibit human platelet aggregation induced by several agonists such as ADP, collagen, thrombin, arachidonic acid and adrenaline in platelet rich plasma (PRP), in a reversible and concentration-related manner (Ferreira et al., 1999). In addition, this extract was demonstrated to have antioxidant, analgesic and anti-inflammatory properties in vivo (Ferreira et al., 2003, 2004). This fraction had no overt toxicity when administered orally to mice.
The phytochemical investigation of A. oncocalyx heartwood extracts has resulted in the isolation and characterization of several uncommon terpenoid quinones and hydroquinones with a C16 backbone (Pessoa et al., 1993, 1995; Marques et al., 2000). Two of these secondary metabolites, named oncocalyxone A (OncoA) and C were obtained in significant amounts. Both compounds were shown to have antitumour activity (Leyva et al., 2000; Pessoa et al., 2000) possibly related to their antimitotic effects (Lotufo et al., 2002) and it was subsequently shown that, in primary cultures of human lymphocytes, OncoA is devoid of genotoxicity (Pessoa et al., 2003).
More recently, we demonstrated that OncoA (Figure 1) is the active principle of A. oncocalyx responsible for its platelet antiaggregatory activity and that this compound does not have hypotensive activity in vivo nor does it affect the perfusion pressure of the vascular mesenteric bed perfused at constant flow and precontracted with phenylephrine (Sousa et al., 2002). The aim of the present study was to evaluate the mechanism of antiaggregatory activity in platelets induced by OncoA isolated from A. oncocalyx.
Figure 1.
Chemical structure of oncocalyxone A (OncoA).
Materials and methods
Isolation of OncoA from A. oncocalyx
Auxemma oncocalyx Taub was collected at Pentencoste City in the state of Ceará, which is located in Northeast Brazil. The species was identified by Professor Afrânio Gomes Fernandes from the Department of Biology of the Federal University of Ceará where a voucher was deposited at the Prisco Bezerra Herbarium under the registration number 18459.
OncoA was isolated as a deep red powder, mp 207–208 °C, from the ethanol extract of the heartwood of A. oncocalyx as previously described by Pessoa et al. (1993). The structure of this secondary metabolite was unambiguously established by spectrometric techniques such as IR, MS and a combination of 1D and 2D NMR methods. The structure was deduced as rel-8α-hydroxy-5-hydroxymethyl-2-methoxy-8αβ-methyl-7,8,8a,9-tetrahydro-1,4-anthracenedione, which was named as Oncocalyxone A.
Evaluation of the effects of OncoA on platelet aggregation in vitro
The study was approved by the Butantan Institute Review Board and the Brazilian National Council. Platelets were isolated from the blood of healthy non-smoking human volunteers (seven men and 22 women), from 18 to 45 years of age (average of 25 years). The smokers were excluded because smoking is known to increase both platelet adhesiveness and aggregation (Law and Wald, 2003; Leone, 2005). Only volunteers who had not taken medicines at least 2 weeks before blood collection were included. Blood samples were taken from the antecubital vein and directly drawn into plastic tubes containing tri-sodium citrate (3.8%). Platelet rich plasma was prepared by centrifugation of blood samples at 180 g for 10 min. After removal of the PRP, platelet poor plasma (PPP) was obtained by centrifugation of the remaining blood sample for 20 min at 500 g. Platelets in PRP were counted by using a cell counter (System 9020 AX, Serono Diagnostics, Allentown, PA, USA), and the number adjusted to 3 × 108 platelets mL−1 with homologous PPP. All the assays were performed immediately after venepuncture and processing of samples and all protocols were performed no more than 3 h after blood collection to avoid loss of platelet sensitivity.
A platelet aggregation test was performed using the turbidimetric method described by Born and Cross (1963) on a lumi-aggregometer (modelo 400 VS, Chrono-Log, USA) with recording and analysis of data made by the Aggrolink software (Chrono-Log, USA). Aggregation was measured at 37 °C under continuous stirring at 1000 r.p.m. in 450 μL aliquots of PRP or washed platelets with data being stored in the computer for analysis. Platelet aggregation was measured by light transmission, with 100% calibration as the absorbance of PPP and 0% calibration as the absorbance of PRP. The aggregation was measured by the height of aggregation curves after 10 min of incubation with specific agonists.
Platelets were stimulated in PRP with ADP (10 μM), collagen (2 μg mL−1), AA (500 μM) or ristocetin (1 mg mL−1) and in suspensions of washed platelets with thrombin (5 nM) (n=6 for each agonist). To determine the effect of OncoA on platelet aggregation, samples of PRP or washed platelets were incubated for 5 min with either vehicle (0.5% DMSO in saline; v/v) or OncoA (32, 64, 128 or 256 μM) before addition of the specific agonist.
To test the reversibility of OncoA-induced platelet aggregation inhibition, PRP was incubated with the quinone (256 μM) or vehicle at room temperature for 5 min and thereafter both aliquots were challenged with AA (500 μM). After recording the inhibition induced by OncoA in PRP, the platelets were washed and incubated again with quinone or vehicle during the same time and then stimulated with AA.
Investigation of the role of thromboxane A2 in inhibition of platelet aggregation by OncoA
In another set of experiments, the blood samples were collected from volunteers that had taken aspirin (500 mg) the night before to irreversibly block TXA2 synthesis (n=4). In these samples, aggregation was evoked by collagen (8 μg mL−1), which induced aggregation by a TXA2-independent mechanism (Siess, 1989; Herman, 1998) in PRP and the curves of aggregation were compared between vehicle and OncoA (32, 64, 128 or 256 μM).
Evaluation of the role of soluble guanylate cyclase (sGC) and PDE5 in the OncoA-induced inhibition of platelet aggregation
Cyclic GMP and some PDE5 inhibitors are known to be involved in the antiplatelet activity of several agents, for example sodium nitroprusside (SNP) and dipyridamole (Geiger, 2001). Therefore, the potential involvement of sGC in the OncoA-induced platelet aggregation inhibition was investigated in PRP using ADP as an agonist (n=6). The effects of SNP (80 μM) a NO-donor drug or OncoA (256 μM) were studied in the absence and in the presence of oxadiazolo[4,3-a]quinoxalin-1-one ODQ (10 μM), a specific inhibitor of the NO-sensitive site of sGC.
Samples of PRP (450 μL) were incubated at room temperature in the following conditions. In an initial experiment, PRP was incubated with OncoA for 5 min, SNP for 3 min or ODQ for 10 min before challenge with ADP (10 μM). In a second set of experiments, samples were treated first with ODQ (incubated for 10 min) and then treated with OncoA for 5 min or SNP for 3 min before ADP challenge. In a third set of experiments, a comparison was made between the effects of SNP (1 μM) on ADP-induced platelet aggregation in PRP either in the presence or absence of OncoA (32 μM) or sildenafil citrate (10 μM) (n=6 for each protocol), a known specific PDE5 inhibitor. Here, samples of PRP were incubated at room temperature with OncoA for 5 min or sildenafil for 5 min or SNP for 3 min before challenge with ADP (10 μM). Finally, in a fourth set of experiments, samples were pretreated first with OncoA (5 min) or with sildenafil (5 min), then with SNP (3 min) before ADP challenge.
Evaluation of effects of OncoA on the platelet release of ATP
The release of ATP from stimulated platelets was measured using the bioluminescence method described by DeLuca and McElory (1978) on a lumi-aggregometer (model 400 VS, Chrono-Log, USA) with Aggrolink package (Chrono-Log, USA). Briefly, the method is based on the formation of an enzymatic complex (luciferin-luciferase in the presence of O2 and ATP) emitting luminescence, which is recorded in parallel to platelet aggregation. To calibrate the lumi-aggregometer the optical density was adjusted to 100% with PRP and to 0% with PPP.
The effect of OncoA (256 μM) on the release of ATP (n=4 for each agonist) was determined in PRP, using as agonists ADP (2.5 μM), collagen (1 μg mL−1) or AA (750 μM). The ATP release reaction was monitored by bioluminescence changes, with luciferin and luciferase (2 mg mL−1) and the concentration of ATP calculated by comparison with an ATP (1.8 μM) standard.
NO determination in washed platelets
NO is a potent endogenous platelet antiaggregatory agent, which can be released by exogenous materials with anti-aggregatory effects. For instance, tetramethylpyrazine increases NO release and thus leads to subsequent antiaggregation (Sheu et al., 2000). To test whether OncoA would increase NO release, the effect of OncoA on NO production was determined (n=4) by chemiluminescence in a suspension of washed platelets as described by Sheu et al. (2000). Briefly, samples of washed platelets (109 platelets mL−1) were incubated with vehicle, OncoA (64, 128 or 256 μM) or collagen (10 μg mL−1) for 15 min at 37 °C. Afterwards, the samples were centrifuged at 328 g for 5 min and the supernatant was separated and deproteinated with ice-cold 95% ethanol for 30 min at 4 °C. The mixture was then centrifuged at 283 g for 7 min and the supernatant kept at −80 °C until the assay was performed.
The test was performed by adding 10 μL of the sample to a reaction chamber with 0.8% vanadium chloride in HCl (1 M) as a reducing agent to convert nitrate to NO, which itself was swept by a helium carrier gas and analysed in a Sievers NO Analyzer (Sievers 280 NOA, Sievers Inc., Boulder, CO, USA). The concentration of NO was calculated by comparison with standard sodium nitrate solutions.
Determination of platelet cAMP and cGMP levels
The intracellular levels of cAMP or cGMP were measured in PRP (2 × 108 platelets/sample) in both ADP (10 μM) activated and non-activated platelets. Non-activated platelets were incubated for 5 min with either vehicle or OncoA (256 μM) or prostacyclin (PGI2; 0.1 μg mL−1). PGI2 was used as positive control because it increases intracellular cAMP. ADP-activated platelets were incubated for 5 min with vehicle or OncoA (256 μM).
Afterwards, EDTA (4 mM) was added to the sample, which was then centrifuged at 450 g for 2 min. The supernatant was disposed, and the remaining cell pellet was resuspended and incubated with 300 μL of HCl (0.1 M). After incubation, platelet lysis was confirmed by light microscopy (LABO PHOT, Nikon, Japan) and samples were centrifuged at 350 g for 10 min and the lysate was subsequently processed according to the manufacturer's instructions for immunoassay determination of intracellular cGMP or cAMP (Assay Designs, Ann Harbor, MI, USA).
Flow cytometry studies: interaction of OncoA with platelet receptors
The effect of OncoA on different receptors expressed on the surface of platelets and involved in platelet aggregation was also tested. Samples (20 μL) of PRP (3 × 108 platelets mL−1) were incubated with vehicle or different concentrations of OncoA at 37 °C for 5 min (n=4 for each protocol). Then, samples (3 μL) were transferred to a polystyrene tube containing 40 μL of fetal bovine serum 10% in phosphate-buffered saline pH 7.4 and 2.5 μL of monoclonal antibody. Monoclonal antibodies against the following antigens were used: CD42b (glycoprotein Ibα from GP Ib/V/IX complex, von Willebrand factor receptor), CD41a (glycoprotein IIb from GP IIbIIIa complex, fibrinogen receptor), CD62P (P-selectin). Integrin α2β1, a collagen receptor was marked with CD49b (α2-subunit of α2β1) and CD29 (β1-subunit of α2β1). After 30 min incubation at room temperature the reaction was stopped by addition of 1% paraformaldehyde (300 μL) and flow cytometry was performed using a FACScan cytofluorimeter Becton-Dickison with CELL Quest package (Becton-Dickinson, Mountain View, CA, USA).
Statistical analysis
Data were expressed as mean±s.e.mean. The concentration required to produce 50% inhibition of platelet aggregation (IC50) was calculated using non-linear regression by means of GraphPad 3.0 software and was expressed with its respective 95% confidence intervals. The data was analysed for statistical differences by using repeated measures one-way ANOVA with the correction of Tukey–Kramer as a post hoc test with 95% confidence. The statistical analysis for the platelet ATP-releasing protocol was done using a two-tailed paired Student's t-test with significance level set at 5%.
Drugs and reagents
Arachidonic acid (AA), thrombin, dimethyl sulphoxide (DMSO), ATP, luciferin-luciferase, prostacyclin (PGI2), SNP, 1H-[1,2,4] (ODQ) were obtained from Sigma Chemical (Saint Louis, MO, USA); ADP, collagen and ristocetin from Chrono-Log (Havertown, PA, USA); sildenafil citrate from Pfizer (New York, NY, USA); cAMPc and cGMP enzyme immunoassay kits from Assay Designs (Ann Harbor, MI, USA) and monoclonal antibodies to CD42b, CD41a, CD62P, CD49b, CD29 adhesion molecules from PharMingen (Franklin Lakes, NJ, USA).
The buffer solution (pH 6.5) used to wash platelets had the following composition (mM): NaCl 140.0, NaHCO3 10.0, KCl 2.5, Na2HPO4 0.49, MgCl2 1.0, tri-sodium citrate 22.0, bovine serum albumin 52.2. The Tyrode/Ca2+ solution (pH 7.4) had the following composition (mM): NaCl 134.0, KCl 2.9, Na2HPO4 0.34, MgCl2 1.0, NaHCO3 12.0, CaCl2 1.0 and HEPES 10.0.
All reagents and salts used were from analytical grade and obtained from Vetec (Rio de Janeiro, RJ, Brazil).
Results
Effects of OncoA on platelet aggregation in vitro
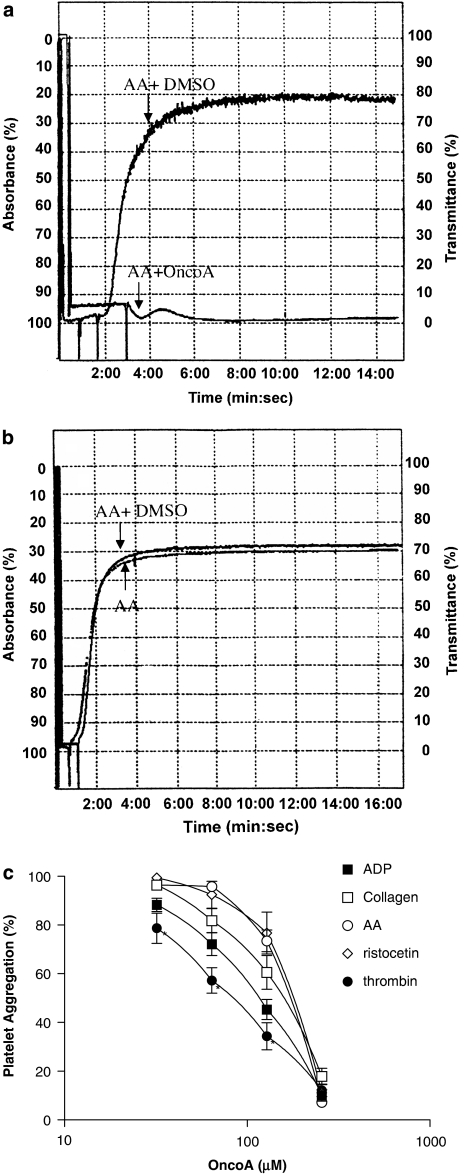
The mean aggregatory response of all agonists were between 70–90%. A typical example of aggregation, with AA (500 μM) is shown in Figure 2a. This Figure also shows a representative recording of the anti-aggregatory effect of OncoA on AA-induced aggregation. The reversibility of this effect is shown in Figure 2b.
Figure 2.
Representative recordings showing the effect of OncoA (256 μm) or DMSO on AA-induced platelet aggregation (a) and the reversal of this antiplatelet activity after washout (b). OncoA inhibits human platelet aggregation induced by ADP, collagen, AA, ristocetin or thrombin (c). Samples of PRP or washed platelets were incubated with vehicle or OncoA (32–256 μm) at room temperature for 5 min before addition of agonist. The heights of aggregation curves were measured at peak effect and expressed as percentage of the control. Results are the mean±s.e.m. of six experiments (*P<0.05 vs collagen, acid arachidonic (AA) and ristocetin, ANOVA for repeated measurements followed by Tukey–Kramer for multiple comparisons).
Pre-incubation of platelets with OncoA (32, 64, 128 and 256 μM) inhibited, in a concentration-related manner, platelet aggregation induced by a range of agonists—ADP (10 μM), collagen (2 μg mL−1), AA (500 μM), ristocetin (1 mg mL−1) and thrombin (5 nM). The result from these experiments are summarized in Figure 2c. From these concentration-effect curves, the calculated IC50 values (μM; mean, 95% confidence intervals) for OncoA were: ADP, 122 [75–199]; collagen, 161 [100–259]; AA, 159 [139–181]: ristocetin, 169 [133–214] and thrombin, 85 [69–105].
Platelet aggregation induced by AA in platelets that were first incubated with OncoA and then washed was similar to that obtained in control samples incubated only with vehicle. This indicates that the effect of OncoA is quite reversible and after washout of OncoA, platelets function normally. For instance, after incubation with OncoA (256 μM) platelet aggregation was inhibited by 96±2.2% (mean±s.e.m. of 4 experiments). After washout, platelets were still able to aggregate by 97±2.4% in relation to control, showing no functional damage induced by OncoA.
Role of thromboxane A2 in the inhibition of platelet aggregation, mediated by OncoA
The inhibition of collagen-induced platelet aggregation by OncoA (32, 64, 128 and 256 μM) was unaltered in platelets where TXA2 synthesis was inhibited by aspirin. The percentage inhibition under these conditions was 16±3.4, 28±4.3, 52±4.6 and 96±0.6%, respectively, with a calculated IC50 of 131 [65–265] μM.
Role of sGC and PDE5 in OncoA-induced platelet aggregation inhibition
To analyse whether sGC or PDE5, were involved in the inhibitory effects of OncoA, specific inhibitors of these pathways were employed. Figure 3 shows that while the inhibition of ADP-induced platelet aggregation by SNP (80 μM) was blocked by 82% in the presence of ODQ (10 μM) (P<0.001, n=6), OncoA-mediated inhibition was not affected (P<0.05, n=6).
Figure 3.
Role of sGC in the inhibition of ADP-induced platelet aggregation by OncoA. Samples of PRP were incubated at room temperature with OncoA (256 μm; 5 min), SNP (80 μm; 3 min) or ODQ (10 μm; 10 min) before ADP (10 μm) challenge. Other samples were treated first with ODQ (10 μm for 10 min) and then treated with OncoA (5 min) or SNP (3 min) before ADP challenge. Data are expressed as mean±s.e.m. of six experiments for each column (***P<0.001 vs SNP alone, ANOVA followed by Tukey–Kramer for multiple comparisons).
On the other hand, OncoA or sildenafil used in concentrations that alone were not able to induce significant inhibition of platelet aggregation (32 or 10 μM, respectively) were both able to potentiate SNP-(1 μM) induced anti-aggregatory activity (P<0.001 with OncoA and P<0.01 with sildenafil) (Figure 4).
Figure 4.
Comparison of the effects of sildenafil (Sild) or OncoA in combination with SNP in ADP-induced platelet aggregation. Samples of PRP were incubated at room temperature with OncoA (32 μm; 5 min) or sildenafil (10 μm; 5 min) or SNP (1 μm; 3 min) before challenge with ADP (10 μm). Other samples were pretreated first with OncoA (5 min) or with sildenafil (5 min), then with SNP (3 min) before ADP challenge. Data are expressed as mean±s.e.m. of six experiments (***P<0.001 vs oncoA or SNP alone;**P<0.01 vs sildenafil or SNP alone, ANOVA followed by Tukey–Kramer for multiple comparisons).
Effect of OncoA on the platelet release of ATP
The release of ATP induced by ADP (2.5 μM), collagen (1 μg mL−1) or AA (750 μM) was strongly inhibited by OncoA (256 μM). The percentage of inhibition was 100% for ADP, 93±4.6% (P<0.01) for collagen and 87±3.6% (P<0.05) for AA, respectively.
Effect of OncoA on NO production by platelets
The effect of OncoA on NO release in platelets was determined in suspensions of washed cells (109 plateletsmL−1). Release of NO on aggregation of washed platelets by collagen was more than double that released from resting platelets (Figure 5). This resting release was not altered by incubation with OncoA even at the highest concentration used (Figure 5).
Figure 5.
Effect of OncoA on NO production in washed platelets. Samples of washed platelets (109 mL−1) were incubated at 37 °C, for 15 min, with vehicle and OncoA (64, 128 or 256 μm). Collagen (10 μg mL−1) was used as positive control. The data are presented as mean±s.e.m. (n=4). ***P<0.001 compared with resting platelets (ANOVA for repeated measures followed by Tukey–Kramer for multiple comparisons). CLG=collagen.
OncoA raised cGMP but not cAMP levels
Exposure of platelets to PGI2 increased platelet cAMP levels 8.6-fold compared to resting platelets (Figure 6a). However, OncoA was unable to modify cAMP concentration either in resting or ADP-activated platelets. On the other hand, OncoA increased cGMP production approximately 12-fold in resting and 11.6-fold in ADP-activated platelets (Figure 6b).
Figure 6.
Effect of OncoA on platelet cAMP (a) or cGMP (b) intracellular concentration. PRP (‘resting platelets') was incubated with vehicle, OncoA (256 μm) or PGI2 (0.1 μg mL−1) at room temperature for 5 min. Other samples (‘ADP-activated platelets') were incubated with vehicle or OncoA before ADP (10 μm) stimulation. The reaction was stopped by HCl (0.1 M) and cAMP or cGMP measured by immunoassay in triplicate. Results are the mean±s.e.m. of four experiments (***P<0.001 vs control, ANOVA for repeated measures followed by Tukey–Kramer for multiple comparisons). R=value obtained in resting platelets; A=value obtained in ADP-activated platelets.
Flow cytometry studies: interaction of OncoA and platelet receptors
The possible interaction of OncoA with GP Ibα from the GP Ib/V/IX complex which is the von Willebrand's factor receptor, was investigated by using a specific monoclonal antibody directed against CD42b. OncoA induced a concentration-dependent inhibition of the binding of this antibody (Table 1). Note however that the same concentrations of OncoA did not affect binding of antibodies to a variety of other platelet surface proteins (CD 41a, 62P, 49b or 29) (Table 1).
Table 1.
Effect of OncoA on GP Ibα (CD 42b), P-selectin (CD62P), GP IIb (CD41a) and GP Ia (CD49b) and GPIIa (CD29)
| Monoclonal antibody |
Fluorescence intensity |
||||
|---|---|---|---|---|---|
| Control |
OncoA (μM) |
||||
| 41 | 82 | 164 | 328 | ||
| Anti-CD42b | 193.8±19.8 | 167.0±13.6# | 125.9±12.5* | 104.2±2.1* | 107.0±1.7* |
| Anti-CD41a | 340.4±26.5 | 333.7±26.3 | 349.8±31.1 | 338.5±24.0 | 337.4±30.0 |
| Anti-CD62P | 17.2±1.3 | 17.6±1.1 | 18.1±0.1 | 20.4±1.6 | 18.0±1.5 |
| Anti-CD49b | 31.6±0.8 | 31.6±0.8 | 30.8±0.8 | 29.8±0.2 | 30.0±1.5 |
| Anti-CD29 | 211.4±9.6 | 215.2±3.7 | 216.7±2.76 | 222.9±6.0 | 217.8±6.9 |
Samples of PRP (3 μL) were incubated at 37 °C, for 5 min, with vehicle or OncoA. Then, 40 μL of 10% bovine fetal serum in phosphate-buffered saline solution, pH 7.4 was added with the specific antibody. After 30 min incubation at room temperature, the reaction was terminated by 1% paraformaldehyde (300μL) and the samples analysed by flow cytometry. Results are the mean±s.e.m. of four experiments.
#P<0.05 vs control; *P<0.001 vs control, ANOVA for repeated measures followed by Tukey–Kramer for multiple comparisons.
Discussion
The search for new antithrombotic agents has resulted in the isolation of many plant constituents with antiplatelet activity, including quinones (Guh et al., 1995; Reis et al., 1999; Wu et al., 2001).
For instance, the anthraquinone Frangulin B isolated from Rhammus formosana, specifically inhibited platelet aggregation induced by collagen and was described as a putative antagonist of the collagen receptor (Teng et al., 1993). Shikonin and other naphthoquinones isolated from Arnebia euchroma, a Chinese herb known for its antithrombotic properties, inhibited rabbit platelet aggregation induced by collagen, AA, platelet-activating factor and thrombin by blocking the hydrolysis of inositol phospholipids (Ko et al., 1995). A synthetic naphthoquinone (NQ 301) inhibited human platelet aggregation induced by collagen, arachidonic acid, thrombin and the calcium ionophore A23187 by increasing intracellular levels of cAMP (Zhang et al., 2001). Another synthetic naphthoquinone (J78) was shown to inhibit rabbit platelet aggregation by inhibition of TXA2 synthesis, TXA2 receptor blockade and suppression of calcium mobilization (Jin et al., 2005).
Quinones are thought to rapidly deplete reduced glutathione levels in various tissues (Di Monte et al., 1984; Brown et al., 1991, Tzeng et al., 1994) and this effect is thought to be related to their antiaggregatory activity (as a primary manifestation of cytotoxicity) (Kim et al., 2001). However, OncoA itself has antioxidant activity both in vitro and in vivo (Ferreira et al., 2001, 2003) and has no pro-oxidant activity (data not published).
In this study, we demonstrated that OncoA inhibited, in a concentration-dependent and reversible manner, at least for arachidonic acid, the platelet aggregation induced by ADP, collagen, arachidonic acid, ristocetin and thrombin. The finding that OncoA inhibited aggregation induced by different agonists suggested that OncoA might interfere with a common step of platelet activation. OncoA also inhibited ATP release showing that this quinone impaired secretion of platelet granule content.
The synthesis of TXA2 is a key step in the platelet response to several agonists and this eicosanoid is one of the most powerful agonists for platelet activation (Siess, 1989; Jin et al., 2005). The inhibition of TXA2 synthesis appears not related to the mechanism of OncoA-induced inhibition of human platelet aggregation, since this quinone inhibited collagen-induced aggregation despite the irreversible blockade of cyclo-oxygenase enzyme induced by aspirin. For this assay, we chose collagen as an agonist because at the given concentration it produces platelet aggregation by TXA2-independent mechanisms (Siess, 1989; Herman, 1998). Even in this condition, OncoA was able to inhibit platelet aggregation in a dose-related manner and we therefore did not pursue this pathway further.
An increase in cytosolic levels of cAMP or cGMP is the most potent endogenous mechanism for the inhibition of platelet activity (Herman, 1998; Schwarz et al., 2001). OncoA was unable to induce an increase in cAMP levels of either resting platelets or ADP-activated platelets, yet this quinone specifically increased cGMP levels, whether platelets were stimulated or not. Increases in platelet cGMP levels could explain the inhibition of both granule secretion and platelet aggregation mediated by OncoA (Haslam et al., 1999).
The effect on cGMP levels observed is not dependent on a NO mechanism since OncoA did not increase NO production in human platelets. In addition, the blockade of the NO-dependent activation of soluble guanylate cyclase by ODQ (Moro et al., 1996) did not affect the antiplatelet activity of OncoA. These data suggest that this quinone might act downstream of the activation of sGC or, alternatively, that it activated sGC by interaction with a different site from that for NO. NO-independent activators of sGC such as YC-1 and BAY 41–2272 have already been described (Ko et al., 1994; Stasch et al., 2001).
Both compounds, that is, YC-1 (Galle et al., 1999) and BAY 41–2272 (Mullershausen et al., 2004), were also shown, besides activation of sGC, to inhibit PDE5 activity. Antiplatelet activity could be derived from a synergistic mechanism of reduced degradation and increased production of cGMP. However, sildenafil, a known PDE5 inhibitor, up to 100 μM had no direct antiaggregatory activity in ADP-activated platelets in our observations. Wallis et al. (1999) showed that sildenafil has no direct effect on the platelet function but potentiated SNP antiaggregatory activity in both rabbit and human platelets. In this regard, we also found that OncoA, used at concentrations with no significant platelet antiaggregatory activity, potentiated the inhibitory action of SNP in human platelet aggregation with an effect quite similar to sildenafil. Hence, like YC-1 and BAY 41–2272, OncoA may directly increase cGMP levels in platelets by a synergistic mechanism, combining increased production and reduced degradation of cGMP.
The glycoprotein complex Ib-V-IX is the receptor involved in the adhesion of non-activated platelets to von Willebrand factor. In this receptor, the GP Ibα subunit is the most important in terms of mass and functional sites. The interference of OncoA in the binding of a specific monoclonal antibody to GP Ibα, as shown by flow cytometry, would explain the inhibition of ristocetin-induced platelet aggregation (Ward et al., 1996). In addition, this receptor is also related to thrombin-induced platelet activation (Jackson et al., 2003) and the interference of antibody-specific binding promoted by OncoA, associated with the induction of cGMP synthesis, clarifies why OncoA is a potent inhibitor of thrombin-induced platelet aggregation. In addition, the binding of OncoA to GP Ibα could evoke downstream effects connecting to activation of sGC at an alternative site to NO, as already noted for YC-1 or BAY41–2272.
Platelet activation and aggregation is essential for primary haemostasis, but is also involved in thrombosis, the leading cause of myocardial infarction and stroke (Ruggeri, 2002). Antiplatelet drugs such as ticlopidine (ADP receptor antagonist) or aspirin were shown to reduce the incidence of stroke in high-risk patients (Hass et al., 1989). Nevertheless, recent reports have shown that adequate antiplatelet effects are not achieved in 5–45% of patients taking aspirin and in 4–30% patients taking clopidogrel (Gum et al., 2001; Eikelboom et al., 2002; Gurbel et al., 2003; Serebruany et al., 2005). These patients are at increased risk of stent thrombosis and cardiovascular complications (Gum et al., 2003; Gurbel et al., 2003; Matetzky et al., 2004; Cuisset et al., 2006). OncoA might be a promising lead compound for antithrombotic drug development, aimed particularly at agents to overcome aspirin and clopidogrel resistance. Synergism or summation of the anti-platelet activity of these drugs is quite possible and deserves further investigation.
Acknowledgments
We thank Dr Avram M Slovic for English revision. Maria Augusta Drago Ferreira is the recipient of a Research Fellowship from the National Research Council of Brazil (CNPq- 150551/2003-7).
Abbreviations
- AA
arachidonic acid
- DMSO
dimethyl sulphoxide
- ODQ
1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one
- OncoA
oncocalyxone A
- PDE5
phosphodiesterase 5
- PGI2
prostacyclin
- PPP
platelet poor plasma
- PRP
platelet rich plasma
- sGC
soluble guanylate cyclase
- SNP
sodium nitroprusside
- TXA2
thromboxane A2
Conflict of interest
The authors state no conflict of interest.
References
- Born GVR, Cross MJ. The aggregation of blood platelets. J Physiol. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PC, Dulik DM, Jones TW. The toxicity of menadione (2-methyl-1,4-naphthoquinone) and two thioether conjugates studied with isolated renal epithelial cells. Arch Biochem Biophys. 1991;285:187–196. doi: 10.1016/0003-9861(91)90348-m. [DOI] [PubMed] [Google Scholar]
- Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006;4:542–549. doi: 10.1111/j.1538-7836.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- DeLuca M, McElory WD. Purification and properties of firefly luciferase. Methods Enzymol. 1978;57:3–15. [Google Scholar]
- Di Monte D, Bellomo G, Thor H, Nicotera P, Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984;235:343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- Ferreira MAD, Leal LKAM, Pessoa ODL, Lemos TLG, Barros SBM, Viana GSB.Antioxidant activity of Quinones isolated from Auxemma oncocalyx Taub VI Pharmatech, Recife 2001183–184.Brazilian Pharmacy Meeting, pp
- Ferreira MAD, Nunes ODRH, Fujimura AHY, Pessoa ODL, Lemos TLG, Viana GSB. Inhibition of platelet activation by quinones isolated from Auxemma oncocalyx Taub. Res Commun Mol Pathol Pharmacol. 1999;106:97–107. [PubMed] [Google Scholar]
- Ferreira MAD, Nunes ODRH, Fontenele JB, Pessoa ODL, Lemos TLG, Viana GSB. Analgesic and anti-inflammatory activities of a fraction rich in oncocalyxone A isolated from Auxemma oncocalyx. Phytomedicine. 2004;11:315–322. doi: 10.1078/0944711041495227. [DOI] [PubMed] [Google Scholar]
- Ferreira MAD, Nunes ODRH, Leal LKAM, Pessoa ODL, Lemos TLG, Viana GSB. Antioxidant effects in the quinone fraction from Auxemma oncocalyx Taub. Biol Pharm Bull. 2003;26:595–599. doi: 10.1248/bpb.26.595. [DOI] [PubMed] [Google Scholar]
- Galle J, Zabel U, Hübner U, Hatzelmann A, Wagner B, Wanner C, et al. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br J Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J.Inhibitors of platelet signal transduction as anti-aggregatory drugs Expert Opin Investig Drugs 200110865–890.Review [DOI] [PubMed] [Google Scholar]
- Guh JH, Ko FN, Jong TT, Teng CM. Antiplatelet effect of gingerol isolated from Zingiber officinale. J Pharm Pharmacol. 1995;97:329–332. doi: 10.1111/j.2042-7158.1995.tb05804.x. [DOI] [PubMed] [Google Scholar]
- Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230–235. doi: 10.1016/s0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
- Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- Haslam RJ, Dickinson NT, Jang EK. Cyclic nucleotides and phosphodiesterases in platelets. Thromb Haemost. 1999;82:412–423. [PubMed] [Google Scholar]
- Hass WK, Easton JD, Adams HP, Jr, Pryse-Phillips W, Molony BA, Anderson S, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- Herman AG. Rationale for the combination of anti-aggregating drugs. Thromb Res. 1998;92:S17–S21. doi: 10.1016/s0049-3848(98)00100-5. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Nesbitt WS, Kulkarni S. Signaling events urderlying thrombus formation. J Thromb Haemost. 2003;1:1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- Jin YR, Cho MR, Ryu CK, Chung JH, Yuk DY, Hong JT, et al. Antiplatelet activity of J78 (2-chloro-3-[2′-bromo,4′-fluoro-phenyl]-amino-8-hydroxy-1,4-naphthoquinone) an antithrombotic agent, is mediated by thromboxane (TX) A2 receptor blockade with TXA2 synthase inhibition and suppression of cytosolic Ca2+ mobilization. J Pharmacol Exp Ther. 2005;312:214–219. doi: 10.1124/jpet.104.073718. [DOI] [PubMed] [Google Scholar]
- Kim SR, Lee JY, Lee MY, Chung SM, Bae ON, Chung JH. Association of quinone-induced platelet anti-aggregation with cytotoxicity. Toxicol Sci. 2001;62:176–182. doi: 10.1093/toxsci/62.1.176. [DOI] [PubMed] [Google Scholar]
- Ko FN, Lee YS, Kuo SC, Chang YS, Teng CM. Inhibition on platelet activation by shikonin derivatives isolated from Arnebia euchrona. Biochem Biophis Acta. 1995;1268:329–334. doi: 10.1016/0167-4889(95)00078-7. [DOI] [PubMed] [Google Scholar]
- Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1 a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- Law MR, Wald NJ.Environmental tobacco smoke and ischemic heart disease Prog Cardiovasc Dis 20034631–38.Review [DOI] [PubMed] [Google Scholar]
- Leone A.Biochemical markers of cardiovascular damage from tobacco smoke Curr Pharm Des 2005112199–2208.Review [DOI] [PubMed] [Google Scholar]
- Leyva A, Pessoa C, Boogaerdt F, Sokaroski R, Lemos TL, Wetmore LA, et al. Oncocalyxones A and C, 1,4-anthracenediones from Auxemma oncocalyx: comparison with anticancer 1,9-antracendiones. Anticancer Res. 2000;20:1029–1031. [PubMed] [Google Scholar]
- Lotufo LVC, Ferreira MAD, Lemos TLG, Pessoa ODL, Viana GSB, Cunha GMA. Toxicity to sea urchin egg development of the quinone fraction obtained from Auxemma oncocalyx. Braz J Med Biol Res. 2002;35:927–930. doi: 10.1590/s0100-879x2002000800010. [DOI] [PubMed] [Google Scholar]
- Marques WB, Dos Santos HS, Pessoa OD, Braz-Filho R, Lemos TL. Anthracene derivatives from Auxemma oncocalyx. Phytochemistry. 2000;55:793–797. doi: 10.1016/s0031-9422(00)00325-3. [DOI] [PubMed] [Google Scholar]
- Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen F, Russwurm M, Friebe A, Koesling D. Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41–2272. Circulation. 2004;109:1711–1713. doi: 10.1161/01.CIR.0000126286.47618.BD. [DOI] [PubMed] [Google Scholar]
- Pessoa C, Silveira ER, Lemos TL, Wetmore LA, Moraes MO, Leyva A. Antiproliferative effects of compounds derived from plants of northeast Brazil. Phytother Res. 2000;14:187–191. doi: 10.1002/(sici)1099-1573(200005)14:3<187::aid-ptr572>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pessoa C, Vieira FM, Lemos TG, Moraes MO, Lima PD, Rabenhorst SH, et al. Oncocalixona A from Auxemma oncocalyx lacks genotoxic activity in phytohemagglutinin-stimulated lymphocytes. Teratog Carcinog Mutagen Suppl. 2003;1:215–220. doi: 10.1002/tcm.10075. [DOI] [PubMed] [Google Scholar]
- Pessoa ODL, De Lemos TLG, De Carvalho MG, Braz-Filho R. Cordiachoromes from Auxemma oncocalyx. Phytochemistry. 1995;40:1777–1786. [Google Scholar]
- Pessoa ODL, De Lemos TLG, Silveira ER, Braz-Filho R. Novel cordiachromes isolated from Auxemma oncocalyx. Nat Prod Lett. 1993;2:145–150. [Google Scholar]
- Reis ALA, Ormelli CB, Miranda ALP, Fraga CAM, Barreiro EJ. Studies on antiplatelet agents from natural safrole. II. Synthesis and pharmacological properties of novel functionalized oxime O-benziylether derivatives. Pharmaceutica Acta Helvetiae. 1999;74:19–28. doi: 10.1016/s0031-6865(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM.Platelets in atherothrombosis Nat Med 200281227–1234.Review [DOI] [PubMed] [Google Scholar]
- Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem Pharmacol. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- Sheu JR, Kan YC, Hung WC, Lin CH, Yen MH. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sciences. 2000;67:937–947. doi: 10.1016/s0024-3205(00)00686-x. [DOI] [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989;69:58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Sousa CM, Nascimento NRF, Lemos TLG, Pessoa ODL, Ferreira MAD.Evaluation of the platelet antiaggregant and vasopressor activity of oncocalyxone A (OncoA) isolated from the quinone fraction of Auxemma oncocalyx Taub XXXIV Brazilian Meeting of the Pharmacology and Experimental Therapeutics Society 2002Brazilian Pharmacology Meeting, Águas de Lindóia; 94Abstract 01.070, pp [Google Scholar]
- Stasch J-P, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- Teng CM, Lin CH, Lin CN, Chung MI, Huang TF. Frangulin B, an antagonist of collagen-induced platelet aggregation and adhesion, isolated from Rhamnus formosana. Thromb Haemost. 1993;70:1014–1018. [PubMed] [Google Scholar]
- Tzeng WF, Chiou TJ, Wang CP, Lee JL, Chen YH. Cellular thiol as a determinant of responsiveness to menadione in cardiomyocytes. J Mol Cell Cardiol. 1994;26:889–897. doi: 10.1006/jmcc.1994.1106. [DOI] [PubMed] [Google Scholar]
- Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Ward CM, Andrews RK, Smith AI, Berndt MC. Mocarhagin, a novel cobra venom metalloproteinase, cleaves the platelet von Willebrand factor receptor glycoprotein Ibα. Identification of the sulfated tyrosine/anionic sequence Tyr-276-Glu-282 of glycoprotein Ibα as a binding site for von Willebrand factor and α-thrombin. Biochemistry. 1996;35:4929–4938. doi: 10.1021/bi952456c. [DOI] [PubMed] [Google Scholar]
- Wu TS, Tsang ZJ, Wu PL, Lin FW, Li CY, Teng CM, et al. New constituents and antiplatelet aggregation and anti-HIV principles of Artemisia capillaries. Bioorg Med Chem. 2001;9:77–83. doi: 10.1016/s0968-0896(00)00225-x. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Chung KH, Ryu CK, Ko MH, Lee MK, Yun YP. Antiplatelet effect of 2-chloro-3-(4-acetophenyl)-amino-1,4-naphthoquinone (NQ301): A possible mechanism through inhibition of intracellular Ca2+ mobilization. Biol Pharm Bull. 2001;24:618–622. doi: 10.1248/bpb.24.618. [DOI] [PubMed] [Google Scholar]