Figure 2.
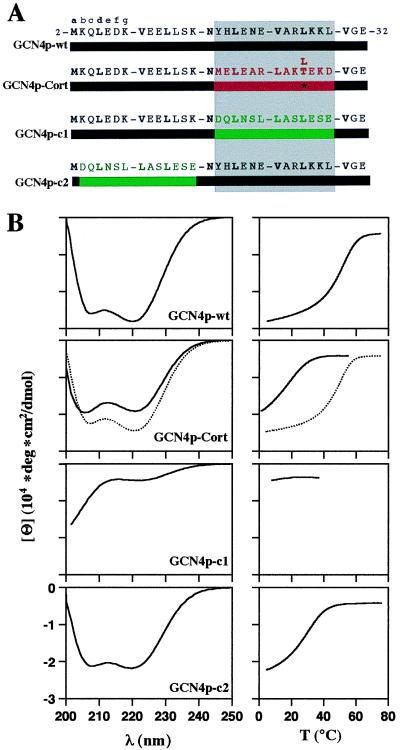
The coiled-coil trigger sequences of GCN4 and cortexillin I are functionally equivalent. (A) Sequences of the recombinant GCN4 polypeptide chain fragments used in this study. GCN4p-wt corresponds to the GCN4 leucine zipper. GCN4p-Cort is a chimeric protein in which the GCN4 trigger sequence has been replaced by the related trigger sequence of cortexillin I (Met-312 to Asp-324) (6, 13). GCN4p-c1 and GCN4p-c2 represent control peptides in which either the GCN4 trigger sequence or the two N-terminal heptad repeats have been replaced by an arbitrarily chosen two-heptad repeat segment from cortexillin I (Asp-270 to Glu-282). Heptad repeats are represented as blocks, and heptad positions are indicated by lowercase letters. The 3,4-hydrophobic repeat with mostly hydrophobic amino acid residues at heptad positions a and d is indicated in bold. Numbers refer to the amino acid positions within GCN4p1 (10). GCN4 sequences are represented in black. The trigger sequence of cortexillin I is shown in red, and the two arbitrarily chosen cortexillin I control heptad repeats are in green. The trigger site critical for coiled-coil formation is indicated by the gray-shaded box. (B) CD spectra (Left) and thermal unfolding profiles (Right) of wild-type and chimeric GCN4 fragments. CD spectra and thermal unfolding profiles of GCN4p-Cort and GCN4p-Cort T/L are represented as solid and dashed lines, respectively. Thermal stability of the proteins was monitored by the CD signal change at 222 nm, [Θ]222. Polypeptide chain concentrations were 75 μM in 5 mM sodium phosphate buffer, pH 7.4, containing 150 mM sodium chloride.
