Figure 3.
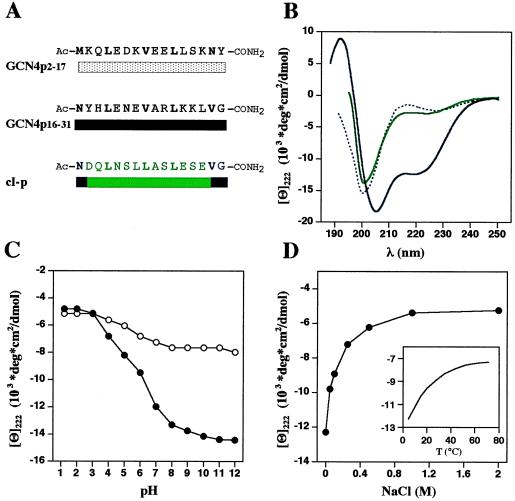
The GCN4 coiled-coil trigger sequence is an autonomous helical folding unit. (A) Synthetic peptide sequences used in this study. GCN4p2–17 and GCN4p16–31 represent N- and C-terminal fragments of the GCN4 leucine zipper. cI-p mainly corresponds to the two arbitrarily chosen cortexillin I control heptad repeats (see Fig. 2). (B) CD spectra of GCN4p16–31 (black solid line), GCN4p2–17 (black dashed line), and cI-p (green line) at 3°C. Peptide concentrations were 30 μM in 1 mM sodium phosphate buffer (pH 7.4). (C) pH dependence of [Θ]222 of GCN4p16–31 at 3°C (•) and 50°C (○) in the absence of sodium chloride. Peptide concentration was 30 μM in 1 mM sodium citrate, 1 mM sodium phosphate, 1 mM sodium borate buffer. (D) The effect of ionic strength (at 3°C) and temperature (without sodium chloride, Inset) on [Θ]222 of peptide GCN4p16–31. Peptide concentration was 30 μM in 1 mM sodium phosphate buffer (pH 7.4). Throughout the pH range and salt concentrations evaluated, helix formation by GCN4p16–31 remained strongly temperature dependent and occurred as a monomolecular reaction.
