Figure 4.
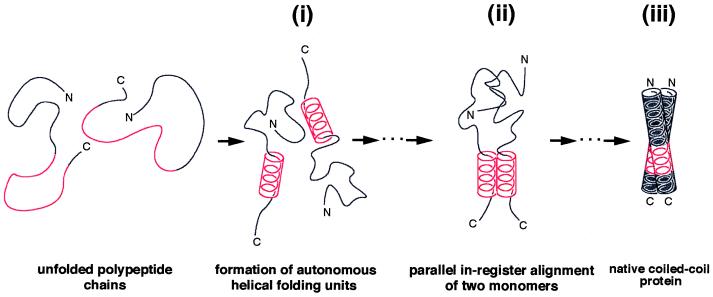
Proposed mechanism of coiled-coil formation in GCN4. Our peptide data (Fig. 3) indicate the presence of a short helical segment within the monomer (i). The fluctuating amphipathic helical stretch corresponds to the autonomous folding unit within the C-terminal half of the GCN4 leucine zipper monomer. Notably, the presence of a helical segment within the monomer significantly limits the number of possible chain conformations and would provide an ideal scaffold for the interaction of critical core residues. Hence, stable dimer formation may involve the interaction of two helical trigger sites at some stage in the folding pathway (ii). Interacting helices then “zip up” along the dimer to finally form the stable coiled-coil structure (iii). It should be noted that such a mechanism ideally would arrange the two-stranded coiled coil in parallel register.
