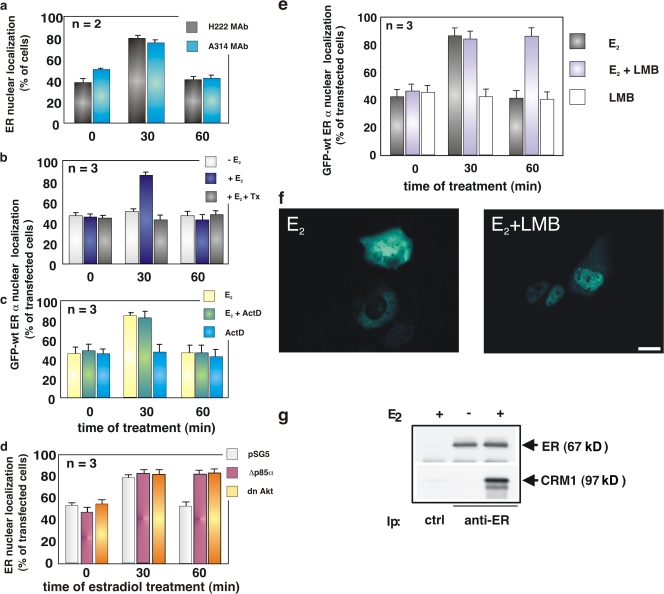
Figure 1.
Estradiol induces nuclear export of ERα, which is regulated by the PI3K–AKT pathway and depends on CRM1. Quiescent MCF-7 cells were used. (a) Cells were untreated or treated with 10 nM estradiol (E2) for the indicated times (min). ERα localization was analyzed by immunofluorescence using the indicated antibodies. (b and c) Cells were transfected with GFP-wtERα then left untreated or treated with the indicated compounds. OH-tamoxifen (Tx; AstraZeneca) was used at 0.1 μM; actinomycin D (Act D) was added at 5 μg/ml, 1 h before estradiol stimulation. GFP-wtERα localization was determined by fluorescence. (d) Cells were transfected with the indicated plasmids and left untreated or treated with 10 nM estradiol for the indicated times (min). The Myc-tagged pSG5, Δp85α, or Myc-His–tagged dominant-negative AKT ectopically expressed in MCF-7 cells were visualized by immunofluorescence, as described in Materials and methods. ERα localization was analyzed by immunofluorescence using the rat H222 anti-ERα mAb. (e) Cells were transfected with GFP-wtERα and then left untreated or treated with 10 nM estradiol in the absence or presence of LMB (at 5 ng/ml). LMB was added 30 min before the hormone. Cells were also treated with LMB in the absence of hormone. GFP-wtERα localization was determined by fluorescence. (a, b, c, d, and e) Cells that fell into the category of exclusively nuclear fluorescence were scored, and data was expressed as a percentage of total cells (in a) or transfected cells (in b, c, d, and e). Data were derived from at least 1,000 scored cells. The results of several independent experiments were averaged; means and SEM are shown. n represents the number of experiments. (f) Images from one experiment in panel e were captured. Panels show GFP-wtERα localization in MCF-7 cells stimulated for 60 min with estradiol (E2) in the absence or presence of LMB. Bar, 5 μm. (g) 35S-labeled HA-CRM1 was incubated with recombinant ERα in the absence or presence of 10 nM estradiol. The purified recombinant RanQ69L (at 1 μM) was included in the incubation mixture of each sample. Proteins were immunoprecipitated with rabbit polyclonal anti-ERα antibody. Eluted proteins were immunoblotted with anti-ERα antibody (top) or revealed by fluorography (bottom).