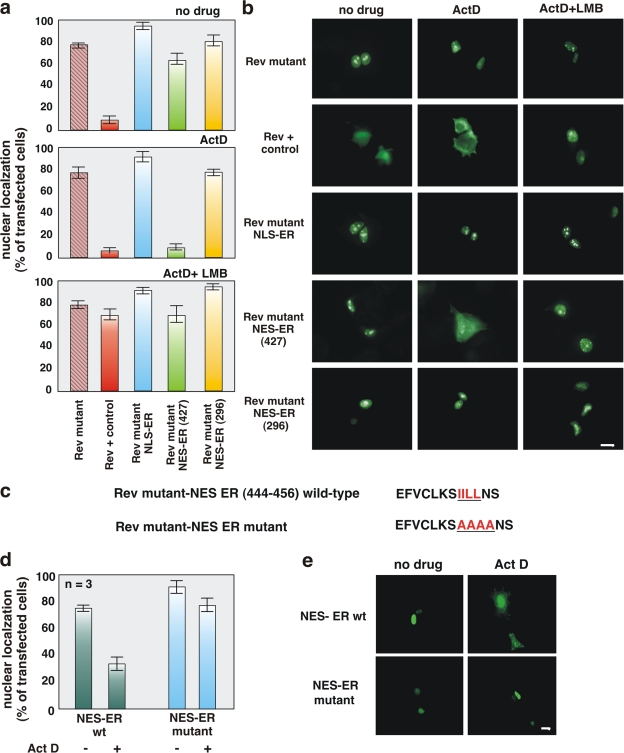
Figure 2.
The ERα 444–456 sequence restores export activity of the NES-deficient REV1.4-GFP. Growing MCF-7 cells were used. (a and b) Cells were transfected with the indicated constructs. After transfection, the cells were left untreated (no drug) or treated with actinomycin D (ActD) at 5 μg/ml, alone or together with 5 ng/ml LMB. The subcellular distribution of GFP proteins was determined by fluorescence microscopy, and cells that fell into the category of exclusively nuclear fluorescence were scored. Data are expressed as a percentage of transfected cells, with mean values taken from at least three experiments. For each experiment, at least 600 cells were scored. (b) Images of one experiment in panel a. (c) The wt ERα 444–456 sequence (NES ERα wt) as well as its mutated version (NES ERα mutant). The putative NES-ERα sequence is indicated by the underlined amino acids, which were substituted with alanine residues in the mutant sequence. The NES-ERα wt as well as the NES-ERα mutant subcloned into the Rev mutant were transfected (d and e) in growing MCF-7 cells. After transfection, the cells were left untreated (−) or treated with 5 μg/ml Act D (+). The percentage of cells with nuclear GFP protein was determined by fluorescence microscopy and graphically shown in panel d. Data were derived from at least 600 scored cells. The results of several independent experiments were averaged. (a and d) Means and SEM are shown. n represents the number of experiments. (e) Images of one experiment in panel d. Bars, 5 μm