Figure 7.
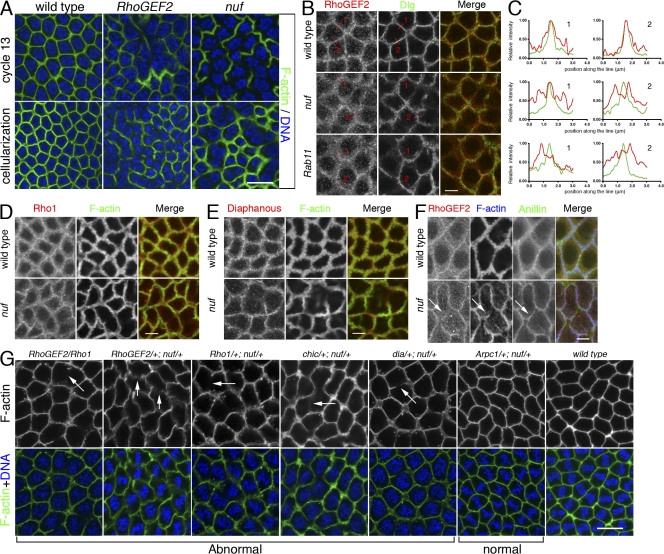
Nuf is required for proper RhoGEF2 recruitment to the furrow. (A) Images of fixed WT, RhoGEF2, and nuf embryos are shown in cycle-13 metaphase and cellularization (staged by DNA, blue) at the sections crossing the furrow. (B) Compared with WT, RhoGEF2 is more diffuse at the furrow in nuf and Rab11 embryos at cycle-13 prophase. Green, F-actin; red, RhoGEF2. (C) Line plots of RhoGEF2 (red curves) and Dlg (green curves) signal intensities (normalized to the maximum signal of each plot into a 0–1 scale) crossing the furrows (red lines in B). WT exhibited a single focused RhoGEF2 peak corresponding to the Dlg peak. In nuf and Rab11 embryos, RhoGEF2 showed unfocused peaks that did not generally correspond with the Dlg peak. (D and E) Rho1 and Diaphanous (red) are not concentrated in furrow regions lacking F-actin (green) but localize properly at regions maintaining F-actin in nuf embryos. (F) At cycle-13 interphase, RhoGEF2 (red) is diffusely localized around the furrow, whereas Anillin (green) is localized normally (arrows). (G) Nuf shows dominant genetic interactions with components of the RhoGEF2–Rho1 pathway. Cycle-13 prometaphase or metaphase embryos (staged by DNA; blue) derived from females with different genetic background were stained with phalloidin to show the furrow structure. (RhoGEF2/Rho1), (RhoGEF2/+; nuf/+), (Rho1/+; nuf/+), (chic/+; nuf/+), and (dia/+; nuf/+) embryos display abnormal furrow morphologies (arrows indicate furrow breaks or weak furrows), whereas (Arpc1/+; nuf/+) embryos have relatively normal furrow morphology. Summary of phenotypic frequencies is shown in Table I. Bars: (A and G) 10 μm; (B–F) 5 μm.