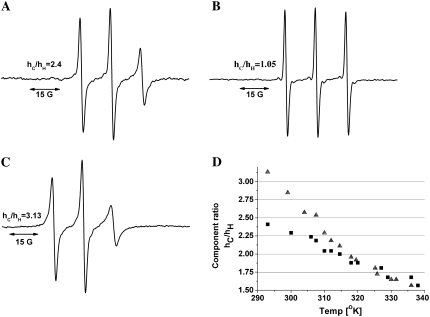
FIGURE 11.
ESR measurements on spin-labeled hNL3-cyt. (A) The ESR spectrum of spin-labeled hNlg3-cyt (in 150 mM NaCl/10 mM sodium phosphate, pH 7.0, at 20°C), reveals relatively sharp peaks that indicate rather limited restriction of the probe's mobility. (B) Disappearance of the relatively slow components, with concomitant appearance of a sharp triplet, when a 100-fold molar excess of DTT was added to the spin-labeled protein. (C) ESR spectrum obtained in the presence of 66% TFE, at 20°C. The peak intensity of the high-field component (hH) in the conjugate (which is very sensitive to label movement) decreases relative to that seen in the absence of TFE, suggesting a decrease in segmental mobility of the polypeptide. (D) Temperature-dependence of the (hC)/(hH) ratio. Both in the absence (▪) and presence of TFE (▴), the ratio decreases with temperature, reflecting an increase in mobility.