Abstract
Since the majority of heroin abusers use injection as the primary route of admission, heroin abuse contributes significantly to the transmission of hepatitis C virus (HCV). We determined HCV infection and its genotype distribution among injection heroin users in Wuhan, the largest city in the central China. Eight hundred seventy eight (84%) out of 1046 serum specimens from the injection drug users were positive for HCV antibody. Out of randomly selected 122 specimens positive for HCV antibody, seventy-eight (64%) had detectable HCV RNA with genotype 6a as the predominant strain (50%), followed by 3b (32.2%), 1a (8.1%), 1b (6.5%), and 3a (3.2%). HCV RNA levels in male heroin users were significantly higher (P = 0.013) than those in the female subjects. Although there was no significant difference in HCV RNA levels among the specimens positive for HCV 6a and 1a/1b, the samples with 6a or 1a/1b contained higher levels of HCV RNA than the specimens positive for HCV 3b (P = 0.019, P = 0.012, respectively). These findings indicate that there is a high prevalence of HCV infection with genotypes 6a and 3b as predominated strains among injection heroin users in Wuhan, China.
Keywords: Hepatitis C Virus, Genotype, Wuhan, China, Injection Heroin Abuser
Hepatitis C virus (HCV) is estimated to chronically infect more than 170 million people worldwide, causing a spectrum of liver disease ranging from an asymptomatic carrier state to end-stage liver disease. While HCV infection through contaminated blood products decreased dramatically due to improved HCV screening tests, injection drug users (IDUs) became the primary source of new HCV infection (Alter, 1997; Alter, 1999; Thomas et al., 1994). IDUs comprise the largest risk group for HCV infection (Alter, 1999; Lauer and Walker, 2001; Mathei et al., 2002). Rates of HCV infection among past and current IDUs are extremely high generally ranging from 70 to over 90% (antibody positive for HCV) in the United States (Diaz et al., 2001; Thomas et al., 1995). Although HCV infection is epidemically worldwide, there is a large degree of geographic variability in its distribution. HCV genotypes have unique patterns of geographic distribution, which is associated with differences in response to interferon treatment (Pascu et al., 2004; Teo and Hayes 2004). Sequence comparisons of variants from different geographical areas have led to the identification and classification of at least six HCV major genotypes, many of which contain a number of more closely related, yet distinct subtypes of the virus (Forns and Bukh, 1999; Stuyver et al., 1994; Wyld et al., 1997).
China is facing an extraordinary re-emerging problem with drug abuse (Liu Z et al., 2006). Injection drug use, predominately heroin injection, has accelerated in China following economic reforms that began in 1979. Although China has a long history of opiate abuse, injection as a route of administration of heroin did not emerge until the late 1980s when the southern borders were opened and there was an increase in the trade with West (McCoy et al., 2001). Drug abuse has become a major problem since then. The total number and geographic locations of drug abusers in China remain unclear. The cumulative number of registered drug users in China has increased from 70,000 in 1990 to 1.14 million in 2004 (Liu Z et al., 2006; Zhao et al., 2004). Many cities and counties across China have reported illegal drug users. Wuhan is a large city in the central part of China, and holds convenient communication with other parts of the country. Although the high prevalence of HCV infection among injection drug users has been reported in many parts of China, there has been no report about HCV infection and its genotype distribution in Wuhan, the largest city in central China where there has been a explosive growth of injection drug use in the recent years.
In order to determine the prevalence of HCV infection and genotypic distribution among injection heroin users in Wuhan city, a total of 1046 serum specimens were collected from injection drug users in the two-detoxification centers in Wuhan city during the period from April 2005 to October 2005. These specimens are collected for the surveillance program of Wuhan CDC that is currently monitoring over 1000 injection drug users in Wuhan city. These specimens are anonymous, and unlinked to any subject identifiers. Out of those specimens positive for HCV antibody, we selected 122 samples positive for HCV RNA to determine HCV viral loads and HCV genotype distribution. HCV antibody in serum was detected by a commercial EIA system (Kehua Biotechnology Inc. Shanghai, China). The total RNA was extracted from 200μl of serum, RNA precipitates were re-suspended in 20 μl of RNase-free water, one tenth of total RNA (2μl) was subjected to reverse transcription (RT) using the 20ul RT System (Promega, Madison, WI). HCV RNA in serum was measured by the real time RT-PCR as described in our earlier study (Yang et al., 2002). HCV RNA+ samples were genotyped by direct sequencing of RT-PCR products representing core- and E1-encoding regions (C/E1) of the HCV genome, location from start of the reference strain H77 genome 843 → 1316. The first-round PCR was performed with a 50 μl reaction volume containing 10ul cDNA, 1×PCR buffer, 0.4 μM primer forward (493S-H77, 5-GCAACAGGGAACCTTCCTGGTTGCTC-3), location from start of the reference strain H77 genome 834 → 859 and 0.4μM primer reverse (987R-H77, 5-CGTAGGGGACCAGTTCATC- ATC AT-3), location from start of the reference strain H77 genome 1306 → 1328 (Ray et al., 2000), 0.2 mM deoxynucleoside triphosphates, 1.5 mM MgCl2, and 1.5 U of Perkin–Elmer AmpliTaq Gold DNA polymerase. All reagents were purchased from Invitrogen (Carlsbad, Calif.). This reaction was incubated at 94°C for 2 min; 30 cycles of 94°C for 15 s, 60°C for 15 s, and 72°C for 30 s; and 72°C for 5 min. The second round of PCR was performed in the same way as the first round except for the primers (502S-H77, 5-AACCTTCCTGGTTGCTCTTTCTCTAT -3, location from start of the reference strain H77 genome 843 → 868 and 975R-H77, 5-GTTCATCATCATATCCCATGCCAT-3), location from start of the reference strain H77 genome 1293 →1316. The amplified products were then analyzed by electrophoresis in a 1.5% agarose gel stained with ethidium bromide. PCR products were purified with Wizard PCR Preps DNA Purification System (Promega, Madison, WI) prior to sequencing to analyze the genotypes of HCV. The sequences were determined in a PRISM version 3100 automated sequencer (ABI, Foster City, CA, USA). The sequences of the specimens were compared with those in the National Center for Biotechnology Information Nucleotide Sequence Database.
Core-E1 sequences (474bp, depending on genotype) were grouped according to genotype, and phylogenetic analysis was performed using the PHYLIP SUITE version 3.5p (PHYLIP, 1993). Nucleotide sequences were aligned by using Clustal X (v.1.83) Program (Jeanmougin et al., 1998) and unrooted phylogenetic trees were constructed by using algorithms from the DNADIST, NEIGHBOR, SEQBOOT, and CONSENSE programs in the PHYLIP. The NJ trees were bootstrapped 1000 times with the SEQBOOT programmed to obtain the final phylogenetic trees. HCV strains were identified as being significantly related if their bootstrap value was ≥70%. The following controls in GeneBank were used to construct the phylogenetic tree: 1a.HC J1 (D00831), 1a.HCV 1 (M62321), 1a.HCV H (M67463), 1b.ARG2 (M74815), 1b.HCV N (AF139594), 1b.HEBEI (L02836), 1c.SR037 (D16191), 2a.AF403230 (AF403230), 2b.DK8 (L16657), 2c.BEBE1 (D50409), 2e.JK020 (D49745), 2i.HN4 (X76415), 2k.VAT96(AB031663), 3a.CB (AF046866), 3a.DK12 (L16630), 3a.K3A (D28917), 3b.HCV Tr (D49374), 3b.ST (D11443), 3b.TH527 (D37839), 3c.NE048 (D16612), 3d.NE274 (D16620), 3e.NE145 (D16618), 3f.NE125 (D16614), 3h.SOM1 (AF216786), 3k.JK070 (D49752), 4a.ED43 (Y11604), 4c.Z6 (L16678), 4d.DK13 (L16656), 4r.1196E1 4 (D43677), 5a.FR741 (D50466), 5a.SA13 (AF064490), 6a.EUHK2 (Y12083), 6a.HK2 (L16634), 6a.VN506 (D88469), 6b.NB56 (AY231583), 6b.TH580 (D37841), 6d.VN235 (D84263), 6e.VN843 (D88478), 6f.TH552 (D37845), 6g.JK065 (D49751), 6h.VN085 (D88466), 6i.TH555 (D37849), 6j.TH553 (D37848), 6k.VN405 (D84264), and 6l.VN530 (D88471).
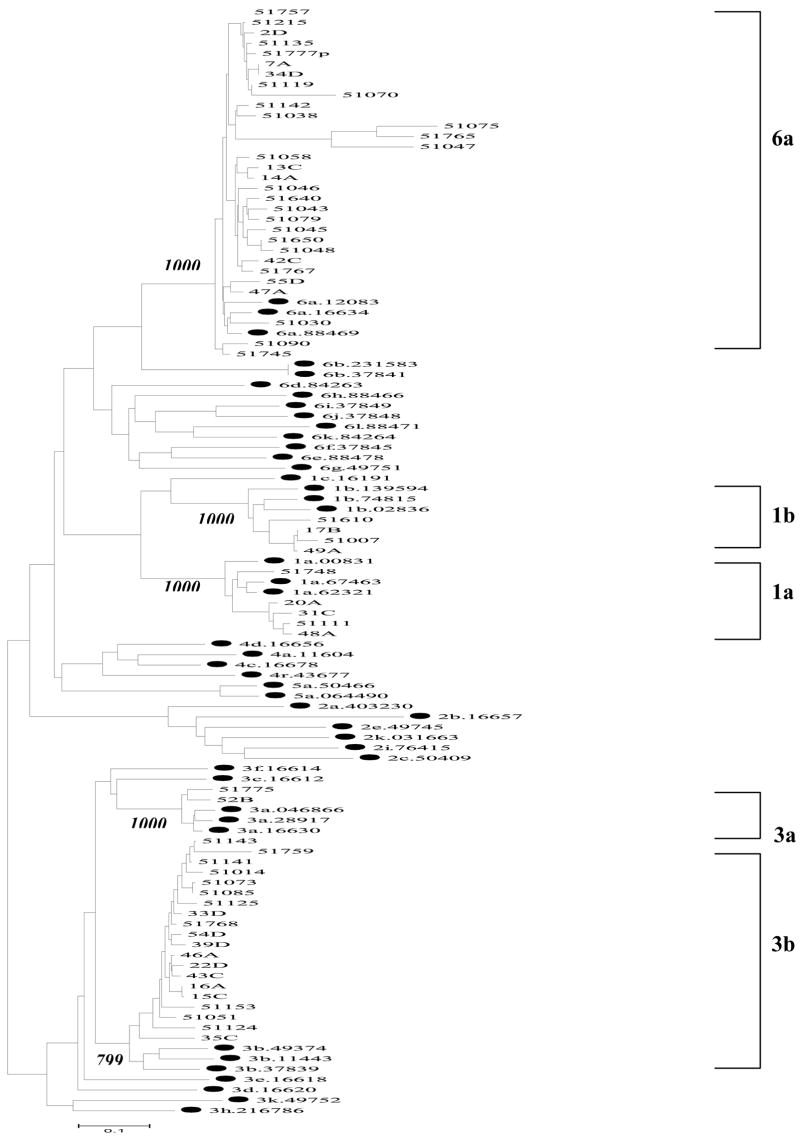
The HCV partial C/E1 nucleotide sequences determined in this study have been deposited in the GenBank sequence database and have been assigned the accession numbers EF185923 to EF185965, EF185967 to EF185971, EF185975, EF185977 to EF185982, EF185985 to EF185988, EF185990, and EF185991. The Statistical Package for the Social Science (SPSS 11.1.0, Inc., Chicago, IL) was used for management and analysis of study data. The differences in distribution of categorical variables were assessed by the Pearson Chi-squared test. The nonparametric Kruskal-Wallis test was utilized to test for differences in HCV viral load among different genotypes and age groups. For comparison of HCV viral load in male and female subjects, duration of drug abuse and needle sharing, the nonparametric Mann-Whitney test was utilized. Eight hundred seventy eight (84%) out of 1046 serum specimens from the injection drug users were positive for HCV antibody. We then randomly selected 122 HCV antibody positive specimens from injection heroin users for HCV RNA analysis by the real-time RT-PCR. Sixty-four percent (78/122) of these specimens had detectable HCV RNA. The mean ± standard deviation (SD) of HCV RNA levels among 78 specimens was 4.48 ± 0.91 log 10 copies/ml. We also analyzed the genotype distribution among those HCV RNA+ specimens. Out of 78 HCV RNA positive specimens, we were able to determine HCV genotypes in 62 specimens using the phylogenetic analysis of a partial HCV C/E1 region (Figure 1). We identified five HCV genotypes (1a, 1b, 3a, 3b and 6a) in these 62 specimens (Table 1), with the predominant genotype 6a (50%), followed by 3b (32.2%), 1a (8.1%), 1b (6.5%), and 3a (3.2%), respectively. We further analyzed the relationship of HCV genotype distribution with other factors. Since sample size of HCV positive specimens for HCV genotypes1a and 1b was too small to be statistically analyzed, we combined all the positive specimens for these two genotypes into one group as 1a/1b. As shown in the Table 1, there is no association between HCV genotype distribution and the factors (gender, age, duration of heroin injection and needle sharing). However, HCV RNA levels in the male heroin users were significantly higher (P = 0.013) than those in the female subjects. The subjects infected with HCV genotype 1 (1a /1b) or 6a had higher levels of HCV RNA than the subjects with HCV 3b, while the sample size for 3a is too small to be analyzed. In addition, the subjects at age of 40 above had higher levels of HCV RNA than other two age groups (Table 2).
Figure 1.
Phylogenetic tree of HCV strains from 70 injecting heroin users at two detoxification centers in Wuhan. The analysis was performed on 474nt within C-E1 region of HCV by using the neighbor-joining method in the PHYLIP package. Bootstrap analysis values (percentages) are shown in italics. The bar at the base of the figure shows the scale for nucleotide substitution per site. Analyzed samples indicated with two digits at the head of a capital letter e.g., 35C, or five digits represented the IDs of samples e.g. 51153; Accession numbers were used for the IDs of the genotype-known reference strains with two digits indicating genotypes at the head of the number (e.g. 3k.D88471) and were labeled by •.
Table 1.
HCV Genotype Distribution and Other Factors
| Prevalence of HCV Genotypes (%)
|
||||
|---|---|---|---|---|
| Factor (No. of Subjects) | 6a | 3a | 3b | 1a or 1b |
| Gender | ||||
| Male (29) | 13 (44.8) | 1 (3.4) | 9 (31.1) | 6 (20.7) |
| Female (33) | 18 (54.5) | 1 (3.1) | 11 (33.3) | 3 (9.1) |
| Age (Year) | ||||
| ≤ 30 (22) | 12 (54.5) | 1 (4.5) | 5 (22.7) | 4 (18.3) |
| 31–40 (22) | 9 (40.9) | 1 (4.5) | 11 (50.1) | 1 (4.5) |
| > 40 (18) | 10 (55.6) | 0 (0.0) | 4 (22.2) | 4 (22.2) |
| Years of Injection Drug Use | ||||
| ≤ 2 (10) | 6 (60.0) | 0 (0.0) | 3 (30.0) | 1 (10.0) |
| > 2 (52) | 25 (48.1) | 2 (3.9) | 17(32.7) | 8 (15.3) |
| Needle Sharing | ||||
| Yes (23) | 11 (47.8) | 0 (0.0) | 8 (34.8) | 4 (17.4) |
| No (39) | 20 (51.3) | 2 (5.2) | 12 (30.7) | 5 (12.8) |
Derived from χ2 tests of association or Fisher’s exact tests comparing HCV genotypes within the risk factors, P > 0.05.
Table 2.
HCV RNA Levels and Other Factors
| HCV RNA (log10copies/ml)
|
|||
|---|---|---|---|
| Variable (No. Subjects) | IQRa | Median | P |
| Gender | |||
| Male (29) | 4.52–5.76 | 5.41 | |
| Female (33) | 3.96–5.16 | 4.6 | 0.013b |
| Age (Year) | |||
| >40 (18) | 4.74–6.00 | 5.59 | |
| ≤ 30 (22) | 4.23–5.20 | 4.73 | 0.019c |
| 31–40 (22) | 3.91–5.49 | 4.42 | 0.028c |
| Needle Sharing | |||
| Yes (23) | 4.09–5.67 | 4.82 | |
| No (39) | 4.17–5.54 | 4.85 | 0.925b |
| Genotype | |||
| 3b (20) | 3.93–4.83 | 4.16 | |
| 6a (31) | 4.24–5.65 | 5.1 | 0.019d |
| 1a,1b (9) | 4.74–6.11 | 5.67 | 0.012d |
Interquartile range
Using Mann-Whitney test, HCV RNA levels of female patients were significantly different (P < 0.05) from male patients.
Using Kruskal-Wallis test, Correction α is 0.025, HCV RNA levels were significantly higher (P < 0.025) in subjects aged above 40 than the subjects at age of 30 or below.
There were only two genotype 3a specimens which can be detected HCV RNA level, we excluded them in our statistics. Using Kruskal-Wallis test, Correction α is 0.025, HCV RNA levels were significantly higher (P < 0.025) in subjects with genotype 6a and 1a,1b infection than subjects with genotype 3b infection.
The present study is the first report about HCV infection and its genotype distribution in Wuhan, the largest city with 8,400,000 residents in central China. When compared with HCV infection rate among IDUs in other parts of China, Wuhan appears to have higher rate (84%) of HCV infection than other cities in China. The studies on injection drug abusers in Shanxi, Chongqing, and Guangxi showed that the seroprevalence of HCV is 7.5%, 44.0%, and 72%, respectively (Fang and Wang, 2001; Pruett et al., 1994; Rebecca et al., 2004). Among 122 randomly selected specimens positive for HCV antibody, seventy-eight (64 %) also were positive for HCV RNA, suggesting that majority of HCV-infected injection heroin users in Wuhan area have chronic infection. This high rate of chronic HCV infection among injection heroin users is consistent with the findings in other developed countries (Alter, 1999; Dore et al., 2003), indicating that injection drug use also is the primary mode of HCV transmission among injection heroin users in Wuhan city.
A genotyping analysis of sixty two HCV RNA positive specimens was able to identify five of six major HCV genotypes with the predominant strain 6a (50%) followed by 3b (32.2%), 1a (8.1%), 1b (6.5%), and 3a (3.2%). This pattern of distribution, however, differs from those reported in other parts of China. In Shanxi, 1b (78.9%) is the predominant genotype of HCV in drug users, which is followed by 2a (15.8%) and 1b/2a (5.3%) (Pruett et al., 1994). The study in Chongqing showed that the HCV 2a and 1b accounted for 46.9% and 33.7%, respectively, while the prevalence of mixed genotype 1b/2a is 19.4% (Fang and Wang, 2001). Interestingly, HCV genotype distribution in Wuhan is consistent with that reported from Guangxi, Guizhou, and Kunming regions (Ding et al., 1999; Lu et al., 2005; Rebecca et al., 2004). In Guangxi, the main HCV genotypes included 6a (38%), 3b (37%), and 1a (19%), whereas genotypes 6e (4%), 3a (2%), and 1b (1%) were present in only a few IDUs. The circulation of uncommon genotype HCV 6a also has been reported in Guizhou (Ding et al., 1999; Rebecca et al., 2004). In Kunming, subtype 3b was found in 33.3% of the samples, while genotypes 6, 1b, and 2a variants were detected in equal numbers (Lu et al., 2005). It is known that HCV genotypes 3 and 6 are common throughout Southeast Asia, especially in Vietnam and Thailand (Verachai V et al., 2002; Wyld et al., 1997), which is geographically close to Guangxi and Kunming, China. In the last decade the Kunming area has become a significant center for trafficking drugs into China and other Asian countries (Beyrer et al., 2000). Since Wuhan is the hinge of traffic and transportation in central part of China, the high prevalence of HCV infection with multiple genotypes among the heroin users in Wuhan city provides direct evidence that Wuhan city may have a key role during the drug trafficking and distribution in the inner parts of China. In addition, because of the unique geographic location of Wuhan city, the “floating” population of heroin users from other parts of China such as Kunming may contribute to the prevalence of predominated strains 6a and 3b in the city.
Another interesting finding of our study is that HCV genotype distribution among female heroin users was not as diversified as that among male subjects. About 90% (30/34) of HCV-infected women in our cohorts were positive for 6a (55.9%) and 3b (35.3%), only 3 out of 34 female subjects were infected with 1a or 1b. In contrast, 7 out of 36 HCV-infected male subjects were positive for 1a or 1b. This difference could be due to the fact that the female heroin users seldom shared syringes with unfamiliar person and strangers. However, since injection drug use was determined by self-reporting, underreporting of injection drug use by the female drug users participated in this study could also contribute to our finding. Nevertheless, this finding supports the studies by others showing that needle/syringe sharing is associated with level of closeness or familiarity among injection partners (Barber et al., 1992; Sherman, 2001). Because of the sample size was small for HCV infection with minor strains (1a, 1b and 3a) among the subjects in this study, the observed differences between male and female should be subjected to future investigations. In consistent with this reported finding that higher HCV RNA levels have been associated with increased age (Castro et al., 2002; Kao et al., 1996; Poynard et al., 2000), our study showed that the subjects aged 40 or above had significantly higher levels of HCV RNA than the younger age groups. Our study also revealed that the viral loads of male patients were significantly higher than female subjects. Some studies have shown that genotype 1b infection of HCV in male subjects was significantly associated with higher viral loads (Lee et al., 2006; Yu et al., 1999). There were significant differences in virus load associated with infection with different genotypes of HCV (Berger et al., 1996; Chan et al., 1995; Lu et al., 1998; Mahaney et al., 1994; Matsumoto et al., 1994; Orito et al., 1994; Yamada et al., 1995). Our finding that HCV RNA levels in the specimens with genotypes 6a or 1a/1b were significantly higher than those positive for genotype 3b provides a possible mechanism for the higher prevalence of HCV infection with genotypes (6a, or 1a/1b) among the subjects of this study.
The high rate infection with HCV 6a strain among the heroin abusers in Wuhan city is troublesome, since studies have shown that the rate of sustained virological response to combination therapy with IFN-α and ribavirin was only 30% for HCV genotype 6 (Lu et al., 2005). In addition, IDUs in China do not routinely have access to the treatment for HCV infection. Thus, it is likely that the HCV strains such as 6a that are highly resistant to the treatment are being transmitted to the majority of drug abuse population (through injection) as well as general population (through sexual activities) in China (Zhang, 2002). Future studies are imperative to develop and implement effective interventions to prevent dissemination of HCV infection in the world’s most populous nation, China.
Acknowledgments
This study was supported in part by the National Institutes of Health grants (DA 12815 and DA 22177 to W.Z.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(3 Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- Alter MJ. Hepatitis C virus infection in the United States. Journal of Hepatology. 1999;31(Suppl 1):88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- Barber JG, Crisp BR, Ross MW, Wodak A. The social behavior of injecting drug users. British Jornal of Social Work. 1992;22:455–462. [Google Scholar]
- Berger AM, Von DP, Doerr HW, Rabenau H, Weber B. Hepatitis C plasma viral load is associated with HCV genotype but not with HIV coinfection. J Med Virol. 1996;48:339–343. doi: 10.1002/(SICI)1096-9071(199604)48:4<339::AID-JMV7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Beyrer RM, Lisam K, Chen J, Liu W, Yu XF. Overland heroin trafficking routes and HIV-1 spread in south and South-East Asia. AIDS Care. 2000;14:75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- Castro FJ, Esteban JI, Juarez A, Sauleda S, Viladomiu L, Martell M, Moreno F, Allende H, Esteban R, Guardia J. Early detection of nonresponse to interferon plus ribavirin combination treatment of chronic hepatitis C. J Viral Hepat. 2002;9:202–207. doi: 10.1046/j.1365-2893.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- Chan CY, Lee SD, Hwang SJ, Lu RH, Lu CL, Lo KJ. Quantitative branched DNA assay and genotyping for hepatitis C virus RNA in Chinese patients with acute and chronic hepatitis C. J Infect Dis. 1995;171:443–446. doi: 10.1093/infdis/171.2.443. [DOI] [PubMed] [Google Scholar]
- Diaz T, Des Jarlais DC, Vlahov D, Perlis TE, Edwards V, Friedman SR, Rockwell R, Hoover D, Williams IT, Monterroso ER. Factors associated with prevalent hepatitis C: Differences among young adult injection drug users in lower and upper Manhattan, New York City. Am J Public Health. 2001;91:23–30. doi: 10.2105/ajph.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Li Y, Tian M. Analysis of hepatitis C virus genotypes in Guizhou area, using second generation line probe assay. Zhong Hua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1999;13:243–246. [PubMed] [Google Scholar]
- Dore GJ, Law MG, MacDonald MHMK. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol. 2003;26:171–184. doi: 10.1016/s1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- Fang YF, Wang YM. Survey of HCV Infection in Intravenous Drug Abusers in Chongqing. Chin J Drug Depend. 2001;10:220–222. [Google Scholar]
- Forns X, Bukh J. The molecular biology of hepatitis C virus. Genotypes and quasispecies. Clinics in Liver Disease. 1999;3:693–716. doi: 10.1016/s1089-3261(05)70234-8. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Science and Justice. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Kao JH, Lai MY, Chen PJ, Hwang LH, Chen W, Chen DS. Clinical significance of serum hepatitis C virus titers in patients with chronic type C hepatitis. Am J Gastroenterol. 1996;91:506–510. [PubMed] [Google Scholar]
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- Lee CM, Lu SN, Hung CH, Tung WC, Wang JH, Tung HD, Chen CH, Hu TH, Changchien CS, Chen WJ. Hepatitis C virus genotypes in southern Taiwan: prevalence and clinical implications. Trans R Soc Trop Med Hyg. 2006;100:767–74. doi: 10.1016/j.trstmh.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lian Z, Zhao C. Drug use and HIV/AIDS in China. Drug Alcohol Rev. 2006;25:173–175. doi: 10.1080/09595230500538835. [DOI] [PubMed] [Google Scholar]
- Lu L, Nakano T, He YS, Fu YS, Haqedorn CH, Robertson BH. Hepatitis C virus genotype distribution in China: predominance of closely related subtype 1b isolates and existence of new genotype 6 variants. Journal of Medical Virology. 2005;75:538–549. doi: 10.1002/jmv.20307. [DOI] [PubMed] [Google Scholar]
- Lu RH, Hwang SJ, Chan CY, Chang FY, Lee SD. Quantitative measurement of serum HCV RNA in patients with chronic hepatitis C: comparison between Amplicor HCV Monitor system and branched DNA signal amplification assay. J Clin Lab Anal. 1998;12:121–125. doi: 10.1002/(SICI)1098-2825(1998)12:2<121::AID-JCLA8>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaney K, Tedeschi V, Maertens G, Dibisceglie AM, Vergalla J, Hoofnagle JH, Sallie R. Genotypic analysis of hepatitis C virus in American patients. Hepatology. 1994;20:1405–1411. doi: 10.1002/hep.1840200605. [DOI] [PubMed] [Google Scholar]
- Mathei C, Buntinx F, van Damme P. Seroprevalence of hepatitis C markers among intravenous drug users in western European countries: a systematic review. J Viral Hepat. 2002;9:157–173. doi: 10.1046/j.1365-2893.2002.00339.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Tanaka E, Suzuki T, Ogata H, Kiyosawa K. Viral and host factors that contribute to efficacy of interferon-alpha(2a) therapy in patients with chronic hepatitis C. Dig Dis Sci. 1994;39:1273–1280. doi: 10.1007/BF02093793. [DOI] [PubMed] [Google Scholar]
- McCoy CB, McCoy HY, Lai S, Yu Z, Wang X, Meng J. Reawakening the dragon: changing patterns of opiate use in Asia, with particular emphasis on China’s Yunnan province. Subst Use Misuse. 2001;36:49–69. doi: 10.1081/ja-100000228. [DOI] [PubMed] [Google Scholar]
- Orito E, Mizokami M, Nakano T, Terashima H, Nojiri O, Sakakibara H, Mizuno M, Ogino M, Nakamura M, Matsumoto Y, Miyata KI, NL JY. Serum hepatitis C virus RNA level as a predictor of subsequent response to interferon-alpha therapy in Japanese patients with chronic hepatitis C. J Med Virol. 1994;44:410–414. doi: 10.1002/jmv.1890440418. [DOI] [PubMed] [Google Scholar]
- Pascu M, Martus P, Hohne M, Wiedenmann B, Hopf U, Schreier E, Berg T. Sustained virological response in hepatitis C virus type 1b infected patients is predicted by the number of mutations within the NS5A-ISDR: A meta-analysis focused on geographical differences. Gut. 2004;53:1345–1351. doi: 10.1136/gut.2003.031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHYLIP FJ. phylogenetic inference package. Version 3.572. Seattle: Department of Genetics, University of Wasington; 1993. [Google Scholar]
- Poynard T, McHutchison J, Goodman Z, Ling MH, Albrecht T. Is an ″ a la carte″ combination interferon alfa-2b plus ribavirin regimen possible for the first line treatment in patients with chronic hepatitis C? Hepatology. 2000;31:211–218. doi: 10.1002/hep.510310131. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Han YC, Wu WJ. A brief review of immunomodulation caused by acute administration of ethanol: involvement of neuroendocrine pathways. Alcohol and Alcoholism Supplement. 1994;2:431–7. [PubMed] [Google Scholar]
- Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL. Genetic epidemiology of hepatitis C virus throughout Egypt. J Infec Dis. 2000;182:698–707. doi: 10.1086/315786. [DOI] [PubMed] [Google Scholar]
- Rebecca J, Garten SL, Zhang JB, Liu W, Chen J, Vlahov D, Yu XF. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. International Journal of Epidemiology. 2004;33:182–188. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- Sherman SG, Latkin CA, Gielen AC. Social factors related to syringe sharing among injecting partners: A focus on gender. Substance Use and Misuse. 2001;36:2113–2136. doi: 10.1081/ja-100108439. [DOI] [PubMed] [Google Scholar]
- Stuyver L, van Arnhem W, Wyseur F, Hernandez E, Delaporte E, Maertens G. Classification of hepatitis C viruses based on phylogenetic analysis of the envelope 1 and nonstructural 5b regions and identification of five additional subtypes. Proc Natl Acad Sci USA. 1994;91:10134–10138. doi: 10.1073/pnas.91.21.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo M, Hayes P. Management of hepatitis C. Br Med Bull. 2004;70:51–69. doi: 10.1093/bmb/ldh022. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Cannon RO, Shapiro CN, Hook EW, 3rd, Alter MJ, Quinn TC. Hepatitis C, hepatitis B, and human immunodeficiency virus infections among non-intravenous drug-using patients attending clinics for sexually transmitted diseases. Journal of Infectious Diseases. 1994;169:990–5. doi: 10.1093/infdis/169.5.990. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Vlahov D, Solomon L, Cohn S, Taylor E, Garfein R, Nelson KE. Correlates of hepatitis C virus infections among injection drug users. Medicine. 1995;74(4):212–20. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Verachai V, Phutiprawan T, Theamboonlers A, Chinchai T, Tanprasert S, Haagmans BL, Osterhaus ADYP. Prevalence and genotypes of hepatitis C virus infection among drug addicts and blood donors in Thailand. Southeast Asian J Trop Med Public Health. 2002;33:849–851. [PubMed] [Google Scholar]
- Wyld R, Robertson JR, Brettle RP, Mellor J, Prescott L, Simmonds P. Absence of hepatitis C virus transmission but frequent transmission of HIV-1 from sexual contact with doubly-infected individuals. Journal of Infection. 1997;35:163–6. doi: 10.1016/s0163-4453(97)91677-7. [DOI] [PubMed] [Google Scholar]
- Yamada G, Takatani R, Kishi F, Takahashi M, Doi T, Tsuji T, Shin S, Tanno M, Urdea MSAKJ. Efficacy of interferon alfa therapy in chronic hepatitis C patients depends primarily on hepatitis C virus RNA level. Hepatology. 1995;22:1351–1354. [PubMed] [Google Scholar]
- Yang JH, Lai JP, Douglas SD, Metzger D, Zhu XH, Ho WZ. Real-time RT-PCR for quantitation of hepatitis C virus RNA. J Virol Meth. 2002;102:119–128. doi: 10.1016/s0166-0934(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Yu ML, Chuang WL, Chen SC, Lin ZY, Hsieh MY, Wang LY, Chang WY. Clinical application of the Quantiplex HCV RNA 2.0 and Amplicor HCV Monitor assays for quantifying serum hepatitis C virus RNA. J Clin Pathol. 1999;52:807–811. doi: 10.1136/jcp.52.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Yang R, Xia XS, Qin SY, Dai JP, Zhang ZB, Peng ZZ, Wei T, Liu H, Pu DC, Luo JH, Takebe YTK, Ben KL. High prevalence of HIV-1 and hepatitis C virus coinfection among injection drug users in the southeastern region of Yunnan, China. Journal of Acquired Immune Deficiency Syndromes. 2002;29:191–6. doi: 10.1097/00042560-200202010-00014. [DOI] [PubMed] [Google Scholar]
- Zhao CZ, Liu ZM, Zhao D, Liu YH, Liang JH, Tang YL, Liu ZY, Zheng JW. Drug Abuse in China. Ann NK Acad Sci. 2004;1025:439–445. doi: 10.1196/annals.1316.054. [DOI] [PubMed] [Google Scholar]