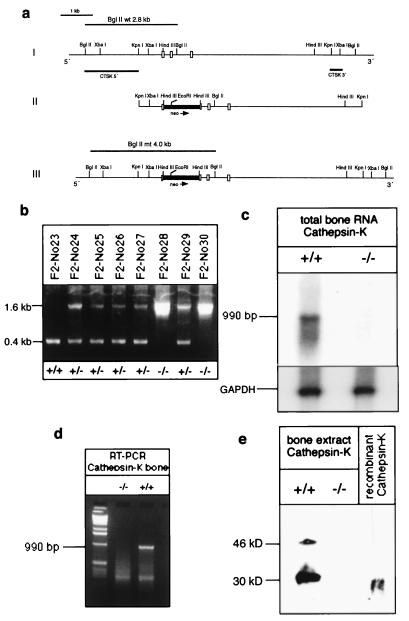
Figure 1.
Targeted disruption of the cathepsin-K gene. (a) Strategy for inactivation of the cathepsin-K gene by homologous recombination in embryonic stem cells. (Part I) 10-kbp portion of the cathepsin-K gene, depicting salient structural information. Exons are indicated by open boxes, flanking introns by solid vertical lines. Horizontal bars, designated as cathepsin-K probes 3′ and 5′, denote the DNA probes used for Southern blot analyses. (Part II) Targeting vector pCK-Kpn(neo) with 5.3-kbp homology to the cathepsin-K gene locus. The neo-cassette was inserted into a HindIII restriction site in exon 7. Arrowhead indicates the direction of transcription of the neo gene. (Part III) Predicted ctsk gene locus after homologous recombination. (b) PCR analysis of tail-genomic DNA with an exon-specific PCR amplifying a 0.4-kb fragment in wild-type (+/+) mice, 0.4-kb and 1.6-kb fragment in heterozygous (+/−) mutants, and a 1.6-kb fragment in homozygous (−/−) cathepsin-K-deficient animals. (c) Northern blot analysis of cathepsin-K expression. Total RNA (10 μg) was hybridized by using murine cathepsin-K and glyceraldehyde-3-phosphate dehydrogenase cDNA probes. (d) RT-PCR of total bone RNA and cathepsin-K-specific primers. In +/+ mice, a 990-bp fragment is amplified, whereas in −/− mice there is no amplification. (e) Western blot analysis of cathepsin-K expression by using an antiserum against mouse cathepsin K (37). In +/+ bone extracts, the 46-kDa cathepsin-K precursor and the 30-kDa mature form are revealed. In −/− bones, no cathepsin-K gene product is apparent. Recombinant mouse cathepsin K served as a control.