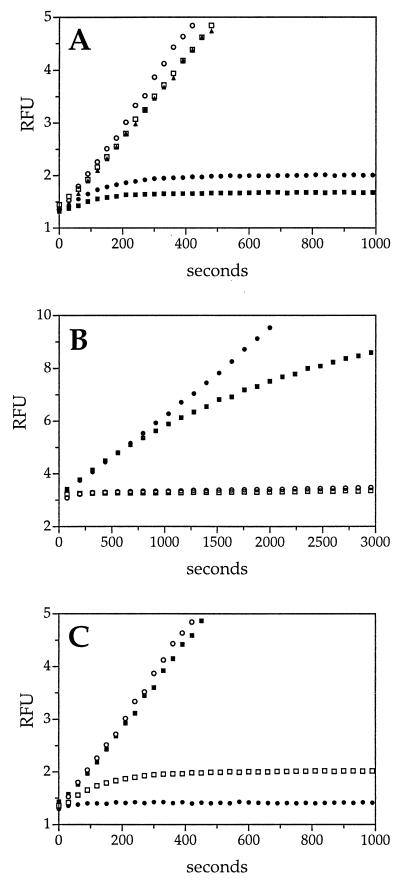
Figure 3.
Kinetic analysis of RSL variable-region mutants. The effect of mutations in the variable region of the RSL on catS inhibition was measured under pseudo-first-order conditions by using the progress-curve method, as described in Fig. 1. Sets of curves from representative experiments are depicted. (A) Loop swap mutants. The rates of product formation by catS in the presence of wild-type SCCA2 (□), the SCCA1(2) mutant (▴) (containing the SCCA2 RSL), wild-type SCCA1 (•), and SCCA2(1) mutant (■) (containing the SCCA1 RSL). CatS without inhibitor (○) served as a control. The rate of catS inhibition by SCCA2(1) was kass = 1.2 × 105 M−1⋅s−1. Concentrations of reagents: inhibitor, 250 nM; enzyme, 10 nM; substrate [(Z-FR)2-R110], 5 μM. (B) P1 mutants. The rate of inhibition of catS by the GlyP2Arg SCCA1 mutant (■) (kass = 1.8 × 104 M−1⋅s−1) and the GlyP2Glu SCCA1 mutant (□) (kass = 1.1 × 105 M−1⋅s−1) was compared with that of wild-type SCCA1 (○). The curve of the GlyP2Glu SCCA1 mutant is obscured by that of the wild-type SCCA1. CatS without inhibitor (•) served as a control. Concentrations of reagents: inhibitor, 360 nM; enzyme, 13.5 nM; substrate [(Z-FR)2-R110], 10 μM. (C) P2 mutants. The rate of inhibition of catS by the PheP3Ala SCCA1 mutant (■) and the PheP3Leu SCCA1 mu tant (•) (kass = 3.3 × 105 M−1⋅s−1) was compared with that of wild-type SCCA1 (□). CatS without inhibitor (○) served as a control. Concentrations of reagents: inhibitor, 250 nM; enzyme, 10 nM; substrate [(Z-FR)2-R110], 5 μM.