Br J Clin Pharmacol 2007; 64: 772–84 (DOI:10.1111/j.1365-2125.2007.03003.x)
Developmental pharmacokinetics of ciclosporin: a population pharmacokinetic study in paediatric renal transplant candidates
S. Fanta, S. Jönsson, J.T. Backman, M.O. Karlsson & K. Hoppu
The authors wish to draw attention to the following errors in one of the tables and in two of the figures that were published with this paper [1]:
In Table 2 the numeric value for the parameter estimate of the inter-individual variability for oral bioavailability, IIV F (CV), should be 0.31.
Also, the text on page 782, paragraph 2, reading ‘Although no factors covaried with the oral bioavailability of ciclosporin, the bioavailability ranged from 10% to 60%, and its IIV was 11%.’ should read ‘Although no factors covaried with the oral bioavailability of ciclosporin, the bioavailability ranged from 10% to 60%, and its IIV was 31%’.
There was a 3-fold error in the Cl/BSA values in Figure 3B and liver volume values in Figure 4. The correct figures and the correct legend for Figure 4 are printed below.
Figure 3B.
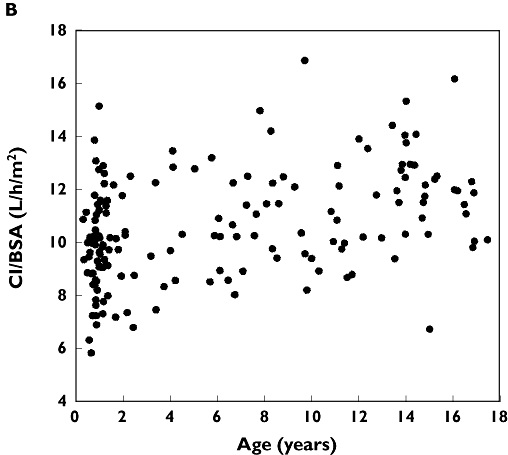
Individual empirical Bayesian estimates of ciclosporin clearance (CL) normalized by body surface area (BSA)
Figure 4.
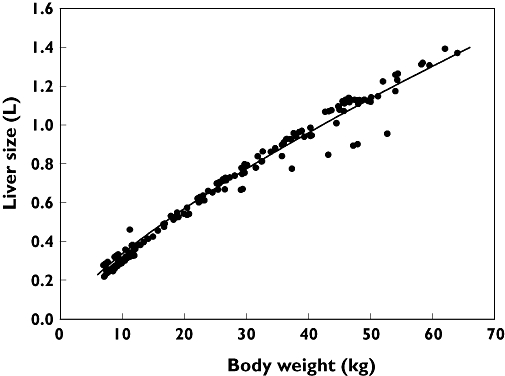
Relationship between body weight and liver size, demonstrated here by the individual liver sizes (•) in our 162 patients calculated with the formula proposed by Johnson et al.[32]: liver volume = 0.722 × BSA1.176; and the allometrically calculated liver size (dark line) based on the data obtained by the previous formula and allometric principles [26]: liver volume = 1.46 × (body weight of child per 70 kg)0.75
REFERENCE
- 1.Developmental pharmacokinetics of ciclosporin: a population pharmacokinetic study in renal transplant candidates. Br J Clin Pharmacol. 2007;64:772–84. doi: 10.1111/j.1365-2125.2007.03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


