Abstract
AIMS
To compare the respirable dose delivery of the hydrofluroalkane fluticasone propionate (HFA-FP) via an optimally prepared Aerochamber Plus spacer (AP), via a Synchro-Breathe (SB) device, and pMDI Evohaler (EH).
METHODS
Seventeen mild to moderate asthmatics completed the study using a randomized, double-blind, double-dummy, three way crossover design. Single doses of placebo or HFA-FP 2.0 mg were administered via the EH, AP, and SB devices. The overnight urinary cortisol : creatinine ratio (OUCC) was measured at baseline and after each dose.
RESULTS
Significant suppression of OUCC occurred from baseline with AP and SB but not EH devices (geometric mean fold suppression, 95% CI): AP: 3.18 (2.29, 4.36), P < 0.001; SB: 1.79 (1.31, 2.40), P = 0.001; EH: 1.12 (0.69, 1.44), p = 0.37 (equating to 68%, 45% and 9% falls, respectively). Significant differences in OUCC between devices were as follows: (geometric mean fold difference, 95% CI): AP vs. EH. 2.83 (2.09, 3.82), P < 0.001; AP vs. SB: 1.78 fold (1.21, 2.60), P = 0.003; SB vs. EH: 1.59 (1.09, 2.31), P = 0.013 (equating to 65%, 44% and 37% differences, respectively).
CONCLUSIONS
The use of an optimally prepared AP spacer and breath actuated SB device, when compared with pMDI, significantly increased the respirable dose of HFA-FP.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Respirable dose delivery of inhaled steroids may be improved by the use of conventional valved holding chambers (such as the Aerochamber Plus spacer), but these are bulky and cumbersome to use.
A novel compact breath actuated device with integrated vortex chamber (Synchro-Breathe) has been developed to overcome these problems,
The lung bioavailability of inhaled fluticasone propionate is dependant on respirable dose delivery, and hence the performance of inhaler devices can be quantified by measuring the degree of adrenal suppression as a surrogate for relative lung dose.
WHAT THIS STUDY ADDS
This study compares the respirable dose delivery (as relative adrenal suppression) of inhaled fluticasone delivered via Synchro-Breathe, conventional pMDI (Evohaler), and an optimally prepared Aerochamber Plus spacer in patients with asthma.
The Aerochamber Plus and the Synchro-Breathe devices produced significantly higher respirable dose delivery of inhaled fluticasone than the pMDI, in terms of the relative degree of adrenal suppression.
Keywords: adrenal suppression, asthma, fluticasone propionate, spacer
Introduction
Inhaled corticosteroids (ICS) are recommended as first line anti-inflammatory therapy in bronchial asthma [1, 2]. The delivery of ICS such as fluticasone propionate (FP) to the lungs by pressurized metered dose inhalers (pMDI) may be facilitated by the use of valved holding chambers such as the 149 ml Aerochamber™ Plus (AP) plastic spacer (Trudell Medical International, London, Canada) [1, 2]. They work on the principle of reducing aerosol velocity and particle size by impaction on the spacer wall along with evaporation, thus causing a reduction in large particle deposition in the mouth and an increase in the amount of fine particles delivered to the lungs. In addition, they reduce local side-effects like oral candidiasis, dysphonia and alleviate synchronization issues between drug actuation and inhalation. It has previously been demonstrated that the use of an optimally prepared 750 ml plastic holding chamber with FP significantly increases the lung bioavailability by 1.94 fold compared with pMDI alone, as assessed by relative suppression of the overnight urinary cortisol : creatinine ratio (OUCC) [3].
Conventional spacers are, however, bulky and cumbersome and not portable and therefore not well accepted by all patients. Moreover such plastic spacers have a high degree of inherent electrostatic charge, which significantly impairs their performance for delivering fine particles [4–6]. A novel palm sized breath actuated spacer device with an integrated vortex chamber (Synchro-Breathe (SB), Vortran Medical Technology Inc, Sacramento, California, USA) has been developed, and is more compact than a conventional valve holding chamber with pMDI, and has none of the problems associated with electrostatic charge (Figure 1). The SB has a reduced actuator orifice diameter, with a unique integrated vortex extension. This creates turbulent ex-valve flow and produces a slower plume velocity when compared with a pMDI alone, thus alleviating any gagging due to throat impaction (so called cold Freon effect). The SB is a unique device which could potentially improve compliance and drug delivery to the lungs, while reducing local adverse effects; however, there are no published in vivo data in asthmatics on its relative lung dose delivery compared with pMDI used alone or in conjunction with a conventional plastic spacer.
Figure 1.
Synchro-Breathe breath actuated integrated vortex spacer device and Aerochamber Plus device with a pMDI (Flixotide Evohaler). The Aerochamber Plus has been optimally prepared and primed with 25 puffs of salbutamol prior to use
The use of ICS is, however, associated with dose dependent systemic side-effects due to systemic absorption from lung and gut (after swallowing) [7]. Fluticasone propionate (FP) exhibits almost complete hepatic first pass inactivation for the swallowed fraction, which along with no appreciable buccal absorption results in negligible oral bioavailability [8]. Thus, the systemic bioavailability of fluticasone depends solely upon lung absorption, which is in turn determined by respirable dose delivery [9]. The respirable dose delivery of FP from different inhaler devices may be reliably measured using suppression of OUCC, as a sensitive surrogate for relative lung dose [3, 10, 11].
We have therefore compared the respirable dose delivery of the hydrofluroalkane (HFA) formulation of FP via an optimally prepared and primed 149 ml Aerochamber™ Plus (AP) plastic spacer, the Synchro-Breathe (SB) device, and a standard pMDI alone (Flixotide™ Evohaler (EH); Allen & Hanburys; Uxbridge, UK), using suppression of OUCC as a sensitive surrogate.
Methods
Patients
Patients with a known diagnosis of stable mild to moderate persistent asthma were recruited from our existing database of research patients, with the following inclusion criteria: age 18–65 years, maintenance dose of ICSs of up to 800 μg of HFA beclomethasone dipropionate (BDP) or equivalent, and forced expiratory volume of 1 s (FEV1) more than 60% of predicted, and a positive methacholine challenge. The Tayside Committee on Medical Research Ethics scrutinized and approved the protocol.
Study design
This was a single centre, randomized study with a double-blind, double-dummy, three way crossover design. At their initial screening visit to the department, participants had their suitability checked against the inclusion criteria and informed consent was obtained. They then entered a structured step down period during which they were withdrawn from their usual maintenance ICS therapy in a manner previously described by our group [12, 13]. Patients were allowed to use salbutamol pMDI, as required throughout, as rescue therapy.
After a minimum 1 week steroid free washout period, they received the following treatments (as single doses) in a randomized, double-blind, double-dummy sequence: (i) eight puffs of placebo HFA FP or active FP by EH (Flixotide™ 250 μg ex-valve per actuation, 220 μg ex-actuator [total dose ex-valve 2 mg, ex-actuator 1.76 mg]); (ii) eight puffs of placebo HFA FP or active FP(total dose ex-valve 2 mg, ex-actuator 1.76 mg) by EH, via an AP spacer device; and (iii) eight puffs of placebo HFA FP or active FP pMDI (total dose ex-valve 2 mg, ex-actuator 1.76 mg), via SB. At each visit, only one of the three treatments was active. The dose of FP used was within the daily dosage range of up to 1.76 mg day−1 as recommended by the manufacturer. However we acknowledge that the dose used does not reflect standard clinical practice for use in mild to moderate patients, and was chosen so as to ensure that an adequate systemic signal, in terms of adrenal suppression, was detectable, to compare differences between devices.
All new pMDIs were primed prior to use by shaking them vigorously and discharging five puffs prior to use. Thereafter, the participants were asked to exhale to residual volume, and the inhaler was actuated at the beginning of inhalation and steady inhalation was maintained until total lung capacity and was followed by breath holding for 10 s or as long as possible. After 30 s, the procedure was repeated for the remaining doses. The pMDI canister was inserted into the SB device and the device was used similar to a conventional pMDI, the main difference being its breath actuation. The AP was prewashed in mild detergent and allowed to drip dry and consequently primed with 25 puffs of salbutamol prior to use to minimize electrostatic charge and optimize respirable dose delivery. It was used with single puffs without any delay between actuation and inhalation, in order to optimize lung dose delivery [4–6]. The package insert instructions regarding device use and drug inhalation were followed [14]. The procedure was repeated for each of the eight inhalations. Correct use of each device was reinforced at each visit prior to drug administration.
Measurements
After completion of the inhalation sequence, the subjects went home with written instructions to collect a 10 h overnight urinary cortisol sample and two sealed urine collection containers. The subjects were asked to empty their bladders at 22.00 h and to collect all of the voided urine until 07.00 h in the first container. A further sample of urine was voided at 08.00 h to complete the 10 h urine collection. The volume of the total sample was recorded, and aliquots were taken for urinary cortisol and creatinine for overnight (10 h) and early morning 08.00 h measurements. A symptom and peak flow diary was reviewed by an investigator at each visit to ensure that their asthma control did not deteriorate significantly whilst in the trial.
Assays
All assays were performed in duplicate in a blinded fashion. The urinary cortisol was measured using a commercial radioimmunoassay kit which had no cross reactivity for FP (DiaSorin Ltd, Wokingham, Berkshire, UK). The intra-assay coefficient of variation was 4% and the interassay coefficient of variation was 8%. Urinary creatinine was measured on a Cobas-Bio auto analyzer (Roche Products, Welwyn Garden City, UK). The intra-assay and interassay coefficient of variation was 4.6% and 3%, respectively.
Statistical analysis
The primary endpoint was overnight cortisol : creatinine ratio. A sample size of 16 completed patients per protocol was chosen to power the study at 80% to detect a 20% difference in overnight urinary cortisol : creatinine ratio, using data from a previous study which detected a 50% difference in OUCC between spacer and pMDI with a sample size of 14 completed patients [3]. Data sets were analyzed for patients who completed the crossover study per protocol. The urinary cortisol : creatinine data were log transformed, and all data were tested for normality prior to analysis. Comparisons were made using an overall analysis of variance, followed by Bonferroni corrected, multiple range testing set with 95% confidence intervals to obviate multiple pair wise comparisons, with the overall α error set at 0.05 (two-tailed). 95% confidence intervals for the differences between treatments were also calculated.
Results
Nineteen participants were randomized into the study and 17 completed the study per protocol (Table 1). One withdrew because of illness; the other withdrew because of a change in personal circumstances.
Table 1.
Patient demographics at baseline
| Patient | Sex | GINA score | Age (years) | ICS dose | FEV1 (l) | FEV1 (% predicted) | Methacholine PC20 (mg ml−1) | OUCC ratio | EMUCC (nmol l−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | I | 57 | 0 | 3.3 | 87 | 8.00 | 117.99 | 187.37 |
| 2 | F | I | 63 | 0 | 1.84 | 90 | 1.64 | 64.66 | 157.52 |
| 4 | M | II | 50 | 0 | 2.60 | 73 | 2.55 | 126.88 | 155.90 |
| 6 | F | I | 21 | 0 | 4.38 | 90 | 1.74 | 52.32 | 165.69 |
| 7 | F | I | 20 | 800 | 3.57 | 105 | 0.22 | 92.94 | 564.87 |
| 8 | F | I | 60 | 100 | 2.47 | 94 | 0.43 | 543.85 | 762.77 |
| 9 | F | I | 31 | 800 | 2.78 | 105 | 0.74 | 65.05 | 79.09 |
| 10 | F | I | 51 | 0 | 2.71 | 93 | 0.11 | 104.09 | 129.59 |
| 11 | F | I | 22 | 200 | 3.18 | 105 | 0.49 | 61.02 | 84.76 |
| 12 | F | I | 22 | 200 | 3.16 | 109 | 2.33 | 73.10 | 234.00 |
| 13 | M | II | 57 | 0 | 1.98 | 61 | 0.04 | 216.54 | 331.49 |
| 14 | F | I | 61 | 800 | 1.79 | 98 | 2.22 | 134.90 | 290.42 |
| 15 | M | I | 21 | 0 | 4.61 | 96 | 4.23 | 116.73 | 50.62 |
| 16 | M | I | 25 | 400 | 5.11 | 114 | 8.00 | 52.48 | 72.31 |
| 17 | M | II | 31 | 500 | 2.11 | 65 | 0.60 | 47.06 | 88.53 |
| 21 | M | I | 41 | 500 | 2.26 | 91 | 0.28 | 83.82 | 142.94 |
| 22 | F | I | 45 | 800 | 2.90 | 97 | 2.59 | 82.47 | 140.59 |
| Mean | 200 | 2.98 | 92.52 | 0.85 | 95.49 | 162.18 | |||
| (SEM) | (0–500)* | (0.23) | (3.55) | (0.34)† | (14.12)† | (28.52)† |
Median (interquartile range).
Geometric mean and (standard error of geometric mean). ICS, beclomethasone dipropionate equivalent dose of inhaled corticosteroid; FEV1, forced expiratory volume in 1 s; FEF25%−75%, forced expiratory flow between 25% and 75%; OUCC, overnight urinary cortisol : creatinine ratio; EMUCC, early morning urinary cortisol : creatinine ratio.
Significant suppression of OUCC and EMUCC occurred from baseline with similar numerical ordering for both end points when comparing the different devices (Table 2). When FP was given via the AP device the geometric mean fold change (95% CI) was 3.18 fold (2.29, 4.36), P < 0.001 and 3.41 fold (1.90, 5.88), P < 0.001, for OUCC and EMUCC, respectively. The SB device significantly suppressed OUCC and a non significant trend with EMUCC was observed: 1.79 (1.31, 2.40), P = 0.001 and 1.66 fold (0.98, 2.78), P = 0.058, respectively. The EH device did not result in significant suppression of either endpoints: 1.12 fold (0.69, 1.44), P = 0.372 and 1.20 fold (0.77, 1.90), P = 0.405, respectively. For OUCC the percentage changes from baseline for AP, SB and EH were 68%, 45% and 9% falls, respectively.
Table 2.
Geometric mean fold suppression from baseline after a single dose of 2000 μg of HFA-fluticasone propionate
| Device | OUCC ratio* (nmol mmol−1) | Geometric mean fold suppression† | 95% CI | Significance P | EMUCC ratio* (nmol mmol−1) | Geometric mean fold suppression† | 95% CI | Significance P |
|---|---|---|---|---|---|---|---|---|
| Aerochamber plus | 29.99 (5.48) | 3.18 | 2.29, 4.36 | <0.001 | 47.86 (15.58) | 3.41 | 1.90, 5.88 | <0.001 |
| Synchro-Breathe | 53.33 (9.25) | 1.79 | 1.31, 2.40 | 0.001 | 97.72 (29.45) | 1.66 | 0.98, 2.78 | 0.058 |
| Evohaler | 84.91 (14.73) | 1.12 | 0.69, 1.44 | 0.372 | 134.89 (37.10) | 1.20 | 0.77, 1.90 | 0.405 |
Geometric mean and (standard error of geometric mean).
Geometric mean fold suppression. FP, Fluticasone propionate; OUCC, overnight urinary cortisol : creatinine ratio; 95% CI, 95% confidence interval; EMF, early morning fraction of urinary cortisol.
Significant differences in OUCC between the devices (Table 3) were demonstrated (geometric mean fold difference, 95% CI). There was a 2.83 fold difference (2.09, 3.82), P < 0.001 between AP and EH; a 1.78 fold difference (1.21, 2.60), P = 0.003 between AP and SB, and a 1.59 fold difference (1.09, 2.31), P = 0.013 between the SB and EH, respectively (equating to 65%, 44% and 37% differences, respectively). The only significant differences for EMUCC were for comparisons between AP vs. either SB or EH.
Table 3.
Between device comparisons of the geometric mean fold difference between primed Aerochamber Plus®, Synchro-Breathe®, and Evohaler® after a single dose of 2000 μg of Fluticasone propionate
| Device | OUCC Geometric mean fold difference* | 95% CI† | Significance P | EMUCC Geometric mean fold difference* | 95% CI † | Significance P |
|---|---|---|---|---|---|---|
| Aerochamber plus vs. Evohaler | 2.83 | 2.09, 3.82 | <0.001 | 2.83 | 1.53, 5.21 | 0.001 |
| Aerochamber plus vs. Synchro-Breathe | 1.78 | 1.21, 2.60 | 0.003 | 2.05 | 1.05, 3.99 | 0.031 |
| Synchro-Breathe vs. Evohaler | 1.59 | 1.09, 2.31 | 0.013 | 1.37 | 0.76, 2.47 | 0.500 |
Geometric mean fold difference.
95% confidence interval of difference. OUCC, overnight urinary cortisol : creatinine ratio; 95% CI, 95% confidence interval; EMUCC, early morning urinary cortisol : creatinine ratio.
Discussion
For the primary outcome of overnight urinary cortisol : creatinine as change from baseline (Figure 2), as a surrogate for relative respirable dose delivery, an optimally prepared and primed AP device resulted in 3.18 fold suppression (i.e. 68% fall), the SB device 1.79 fold suppression (i.e. 45% fall) and the EH 1.12 fold suppression (i.e. 9% fall). This observation was further quantified by comparisons between devices, which indicated that HFA-FP via AP and SB, produced a 2.83 (65% difference) and 1.59 fold (37% difference) greater suppression, respectively, when compared with an EH pMDI. We would point out that in the present study the EH pMDI was being used with optimal technique, which is unlikely to be the case in the real world. Thus, in everyday clinical practice, it is conceivable that the magnitude of differences between AP and SB vs. pMDI would, if anything, tend to be even greater. The increased respirable dose from the SB occurs primarily by slowing down particle velocity and reducing particle size due to the effects of the integrated vortex chamber, while for the AP this occurs by particles colliding with the walls of the holding chamber.
Figure 2.
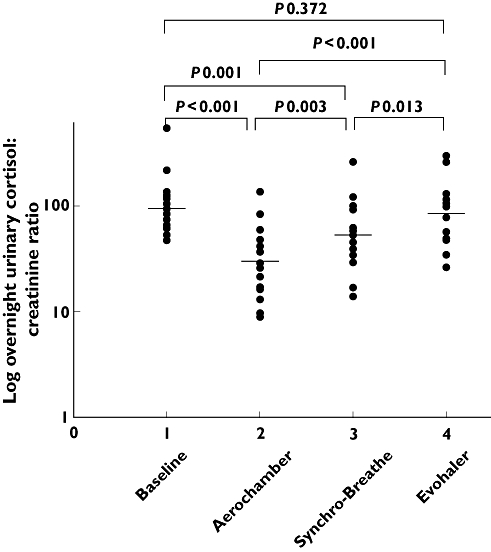
Individual values for overnight urinary cortisol : creatinine ratio with geometric mean at baseline and after inhalation of a single ex-valve dose of 2000 μg HFA-FP via the Aerochamber Plus, Synchro-Breathe, and Evohaler devices plotted on a logged scale
Evaluation of the secondary outcome measure, namely early morning urinary cortisol : creatinine ratio, which is representative of the spot 08.00 h urine fraction, showed a similar pattern but differences from baseline and between devices only achieved significance for the AP and not the SB (Figure 3). For example the relative degree of suppression from the baseline for SB was 1.79 vs. 1.66 fold, respectively, for the overnight and early morning fractions, but was only significant for the former. However the greater variability for the early morning compared with the overnight fractions can be seen by the tighter 95% CIs for within subject differences, which were comparable with the variability for the same outcomes previously reported by Dempsey [3]. This reflects the inherently greater variability in a spot 08.00 h collection vs. an overnight integrated 10 h collection, with the latter having a superior signal to noise ratio for detecting within subject differences between inhaler devices.
Figure 3.
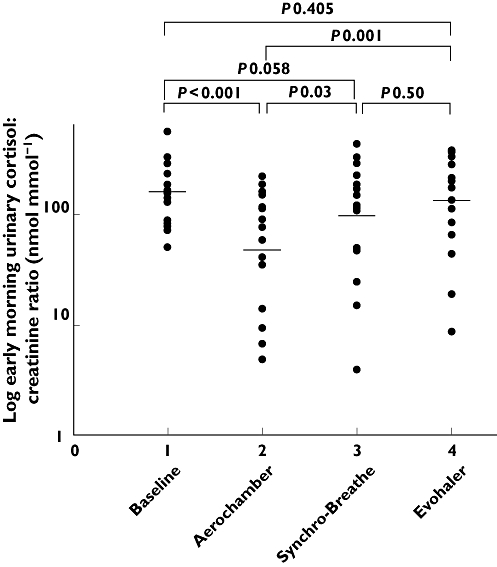
Individual values for early morning urinary cortisol : creatinine ratio with geometric mean at baseline and after inhalation of a single ex-valve dose of 2000 μg of HFA-FP via the Aerochamber Plus, Synchro-Breathe, and Evohaler devices plotted on a logged scale
The AP device, which has a chamber volume of 149 ml, has been shown to reduce oropharyngeal deposition and its bronchodilatory efficacy was similar to an optimally administered pMDI [15]. Similar bronchodilator efficacy results were obtained when the AP device was compared with a Gentlehaler actuator [16]. The Gentlehaler device, which is also an integrated vortex device like the SB (but not breath actuated like SB) has been shown to reduce oropharyngeal deposition and it provides equivalent brondodilator responses when compared with a pMDI [17]. An in vitro impactor study comparing fine particle dose delivery of HFA-BDP via two small volume spacers (Optichamber, 218 ml and AP, 149 ml) and a pMDI revealed no significant differences in respirable particle dose between the pMDI and the optimally prepared AP [18]. In another study looking at the relative potency ratios of inhaled budesonide at doses of 400 μg and 1600 μg day−1 for 2 weeks, the addition of a 750 ml large volume spacer (Nebuhaler) was associated with a two fold greater suppression of early morning serum cortisol with an associated two fold increase in anti-asthmatic efficacy [19]. The combined use of a large volume spacer (Volumatic) with FP via pMDI resulted in a 1.94 fold increase in lung bioavailability, using suppression of overnight urinary cortisol [3]. It is important to recognize that there are significant differences in the in vitro drug delivery characteristics between pMDIs, small and large volume spacer devices with different inhalation drugs [20] and there is a need for in vivo data specifically looking at various drug and device combinations.
When interpreting results of lung bioavailability studies, it is important to recognize that there are several variables which determine the clinical applicability of the results. These include the type of inhaled steroid, the formulation, the actuator device, the optimal use of valve holding chamber or spacer devices, and more importantly the use of correct technique when using a pMDI and spacer combination. In this regard in our study we elected to use the Aerochamber plus in optimal condition with it being prewashed and primed to reduce electrostatic charge, as well as using single puffs without inhalation delay [4–6]. One could always argue that in real life, the performance of a new AP would not be as efficient as we observed in the present study due to lack of priming or use of multiple puffs with delay. In contrast the SB device was used straight out of the box, and unlike an EH pMDI requires little training, due to its breath actuation and the gentle plume emitted from the device. The other advantage of the SB is that it reduces oropharyngeal deposition compared with a pMDI.
Another important factor to take into account is the effect of airway calibre, such that patients with a greater impairment of FEV1 would exhibit reduced lung absorption of fluticasone [21, 22]. Thus, the magnitude of suppression seen in our patients who received a high dose of FP in the presence of preserved airway calibre, would be attenuated if the same dose were given to more severe patients with impaired lung function [23]. However, we believe our results would be valid across a range of asthma severities, because the relative ratios for lung bioavailability when comparing devices would still be similar in more severe patients, even though the absolute degree of suppression for a given device would be less. We did not power our study to look at subgroups according to FEV1%, so we are unable to assess the effect of airway calibre on the degree of suppression with each device. This would also require the use of more severe asthmatics than were enrolled in the present study. We are confident that the 2 mg ex-valve dose used was on the steep part of the dose–response curve for the adrenal suppression, as we were able to show clear separation in terms of the relative degree of suppression with the different devices. In this regard the degree of suppression with the AP in the present study was 68%, which is less than previously reported values of 86% and 90% for the same dose of fluticasone via an optimally prepared large volume spacer [11, 24]. In other words it is evident that there was room for further suppression from the level seen with the AP.
We did not attempt to compare the anti-asthmatic efficacy of the AP and SB devices, as we only gave single doses of FP. In a previous dose ranging study comparing the in vivo efficacy of HFA-BDP via the AP and the Neohaler (which is similar to the SB in also having an integrated vortex chamber), we found no significant differences between devices for effects on either methacholine challenge or exhaled nitric oxide [25]. We realize that an improved lung dose can be a two edged sword, and as seen in the present study, may increase the propensity for systemic adverse effects, such as adrenal suppression, especially with fluticasone during chronic dosing, due to drug accumulation at steady state. One could also argue that increased lung delivery with an AP or SB might be able to facilitate a lower effective maintenance nominal dose after step down. At present prescribing guidelines do not distinguish between the relative lung dose. For example although FP via a large volume spacer delivers a five-fold higher lung dose than from a dry powder inhaler, both devices are recommended for use at microgram equivalent nominal dose [11].
In conclusion, the use of a compact breath actuated integrated vortex device and an optimally prepared AP device both significantly increased the respirable dose delivery of HFA-FP compared with pMDI alone. This increased lung dose could result in increased clinical efficacy. However, there is at the same time a potential for increased systemic adverse effects, unless a lower effective nominal dose is employed. Both of these delivery devices could reduce the incidence of local adverse effects such as oral candidiasis and dysphonia, and obviate problems of poor technique associated with pMDI alone. Eventually, decisions on device selection must be based on an individual basis giving due consideration to a range of factors using an evidence based approach [26]. Further dose ranging studies are now indicated to assess the relative therapeutic ratios of fluticasone delivered via pMDI and SB.
Funding: Unrestricted research grant from the University of Dundee.
REFERENCES
- 1.British guideline on the management of asthma. Thorax. 2003;58(Suppl. 1):i194. doi: 10.1136/thorax.58.suppl_1.1i. 1–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert panel report: guidelines for diagnosis manage asthma update selected topics-2002. J Allergy Clin Immunol. 2002;110(5) Suppl.:S141–219. [PubMed] [Google Scholar]
- 3.Dempsey OJ, Wilson AM, Coutie WJ, Lipworth BJ. Evaluation of the effect of a large volume spacer on the systemic bioactivity of fluticasone propionate metered-dose inhaler. Chest. 1999;116:935–40. doi: 10.1378/chest.116.4.935. [DOI] [PubMed] [Google Scholar]
- 4.Clark DJ, Lipworth BJ. Effect of multiple actuations, delayed inhalation and antistatic treatment on the lung bioavailability of salbutamol via a spacer device. Thorax. 1996;51:981–4. doi: 10.1136/thx.51.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anhoj J, Bisgaard H, Lipworth BJ. Effect of electrostatic charge in plastic spacers on the lung delivery of HFA-salbutamol in children. Br J Clin Pharmacol. 1999;47:333–6. doi: 10.1046/j.1365-2125.1999.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipworth BJ, Lee DK, Anhoj J, Bisgaard H. Effect of plastic spacer handling on salbutamol lung deposition in asthmatic children. Br J Clin Pharmacol. 2002;54:544–7. doi: 10.1046/j.1365-2125.2002.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipworth BJ. Airway and systemic effects of inhaled corticosteroids in asthma: dose–response relationship. Pulm Pharmacol. 1996;9:19–27. doi: 10.1006/pulp.1996.0002. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey OJ, Coutie WJ, Wilson AM, Williams P, Lipworth BJ. Evaluation of the buccal component of systemic absorption with inhaled fluticasone propionate. Thorax. 1999;54:614–7. doi: 10.1136/thx.54.7.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipworth BJ, Seckl JR. Measures for detecting systemic bioactivity with inhaled and intranasal corticosteroids. Thorax. 1997;52:476–82. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RJ, Szefler SJ, Chinchilli VM, Kraft M, Dolovich M, Boushey HA, Cherniack RM, Craig TJ, Drazen JM, Fagan JK, Fahy JV, Fish JE, Ford JG, Israel E, Kunselman SJ, Lazarus SC, Lemanske RF, Peters SP, Sorkness CA. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med. 2002;165:1377–83. doi: 10.1164/rccm.2105013. [DOI] [PubMed] [Google Scholar]
- 11.Wilson AM, Dempsey OJ, Coutie WJ, Sims EJ, Lipworth BJ. Importance of drug–device interaction in determining systemic effects of inhaled corticosteroids. Lancet. 1999;353:2128. doi: 10.1016/S0140-6736(99)01443-9. [DOI] [PubMed] [Google Scholar]
- 12.Fardon TC, Burns P, Barnes ML, Lipworth BJ. A comparison of two extrafine hydrofluoroalkane-134a-beclomethasone formulations on methacholine hyperresponsiveness. Ann Allergy Asthma Immunol. 2006;96:422–30. doi: 10.1016/S1081-1206(10)60909-X. [DOI] [PubMed] [Google Scholar]
- 13.Fardon TC, Lee DK, Haggart K, McFarlane LC, Lipworth BJ. Adrenal suppression with dry powder formulations of fluticasone propionate and mometasone furoate. Am J Respir Crit Care Med. 2004;170:960–6. doi: 10.1164/rccm.200404-500OC. [DOI] [PubMed] [Google Scholar]
- 14.Instructions for use of Aerochamber Plus VHC. 2001. [5 September 2007]. Available at http://www.aerochambervhc.com/physician/instructions.asp.
- 15.Dolovich M, Ruffin R, Corr D, Newhouse MT. Clinical evaluation of a simple demand inhalation MDI aerosol delivery device. Chest. 1983;84:36–41. doi: 10.1378/chest.84.1.36. [DOI] [PubMed] [Google Scholar]
- 16.Chipps BE, Naumann PF, Wong GA, Raabe OG. Clinical comparison of gentle- haler actuator and aerochamber spacer for metered dose inhaler (MDI) use by asthmatics. Respir Care. 1992;37:1414–22. [PubMed] [Google Scholar]
- 17.Newman SP, Clarke SW. Bronchodilator delivery from Gentlehaler, a new low-velocity pressurized aerosol inhaler. Chest. 1993;103:1442–6. doi: 10.1378/chest.103.5.1442. [DOI] [PubMed] [Google Scholar]
- 18.Asmus MJ, Coowanitwong I, Kwon SH, Khorsand N, Hochhaus G. In vitro performance of two common valved holding chambers with a chlorofluorocarbon- free beclomethasone metered-dose inhaler. Pharmacotherapy. 2003;23:1538–44. doi: 10.1592/phco.23.15.1538.31960. [DOI] [PubMed] [Google Scholar]
- 19.Toogood JH, Baskerville J, Jennings B. Use of spacers to facilitate inhaled corticosteroid treatment of asthma. Am Rev Respir Dis. 1984;129:723–9. doi: 10.1164/arrd.1984.129.5.723. [DOI] [PubMed] [Google Scholar]
- 20.Barry PW, O'Callaghan C. Inhalational drug delivery from seven different spacer devices. Thorax. 1996;51:835–40. doi: 10.1136/thx.51.8.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortimer KJ, Harrison TW, Tang Y, Wu K, Lewis S, Sahasranaman S, Hochhaus G, Tattersfield AE. Plasma concentrations of inhaled corticosteroids in relation to airflow obstruction in asthma. Br J Clin Pharmacol. 2006;62:412–9. doi: 10.1111/j.1365-2125.2006.02712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner P, Berar-Yanay N, Davidovich A, Magadle R. Nocturnal cortisol secretion in asthmatic patients after inhalation of fluticasone propionate. Chest. 1999;116:931–4. doi: 10.1378/chest.116.4.931. [DOI] [PubMed] [Google Scholar]
- 23.Lee DK, Bates CE, Currie GP, Cowan LM, McFarlane LC, Lipworth BJ. Effects of high-dose inhaled fluticasone propionate on the hypothalamic-pituitary-adrenal axis in asthmatic patients with severely impaired lung function. Ann Allergy Asthma Immunol. 2004;93:253–8. doi: 10.1016/S1081-1206(10)61497-4. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey OJ, Humphreys M, Coutie WJ, Lipworth BJ. Relative lung delivery of fluticasone propionate via large Volume spacer or nebuliser in healthy volunteers. Eur J Clin Pharmacol. 2001;57:637–41. doi: 10.1007/s002280100363. [DOI] [PubMed] [Google Scholar]
- 25.Menzies D, Nair A, Fardon T, Barnes M, Burns P, Lipworth B. An in vivo and in vitro comparison of inhaled steroid delivery via a novel vortex actuator and a conventional valved holding chamber. Ann Allergy Asthma Immunol. 2007;98:471–9. doi: 10.1016/S1081-1206(10)60762-4. [DOI] [PubMed] [Google Scholar]
- 26.Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, Smaldone GC, Guyatt G. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American college of chest physicians/American college of asthma, allergy, and immunology. Chest. 2005;127:335–71. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]


