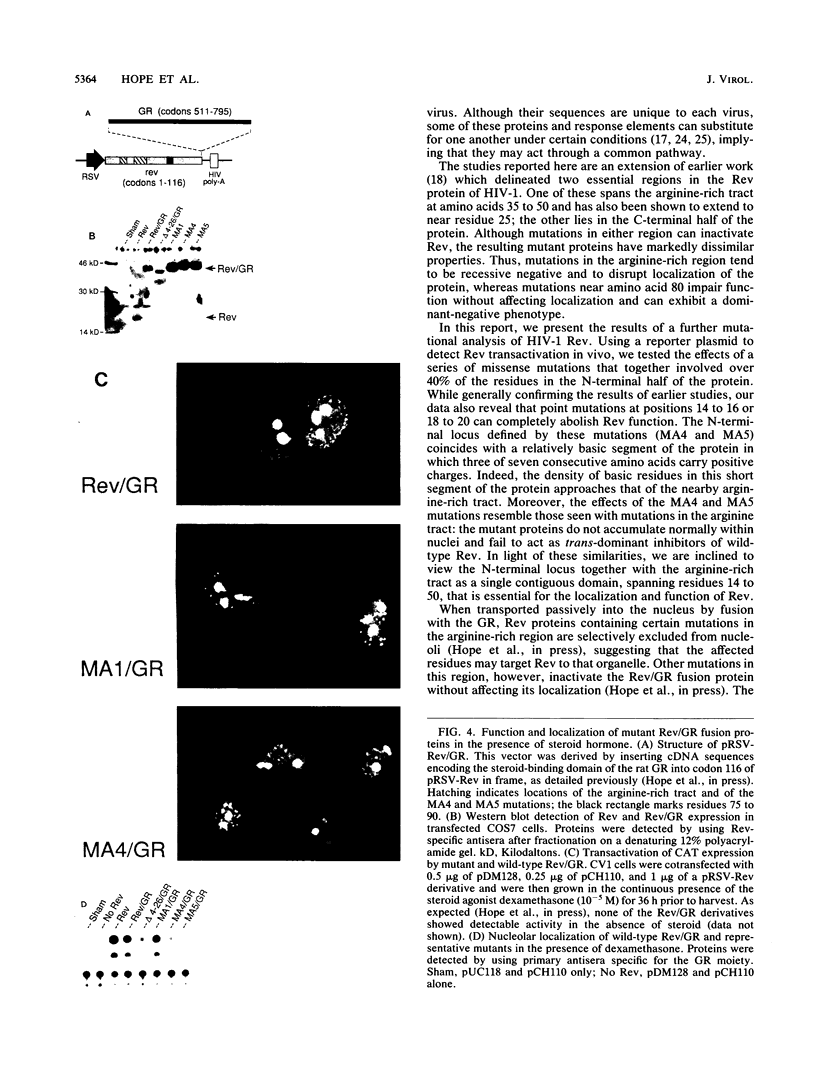
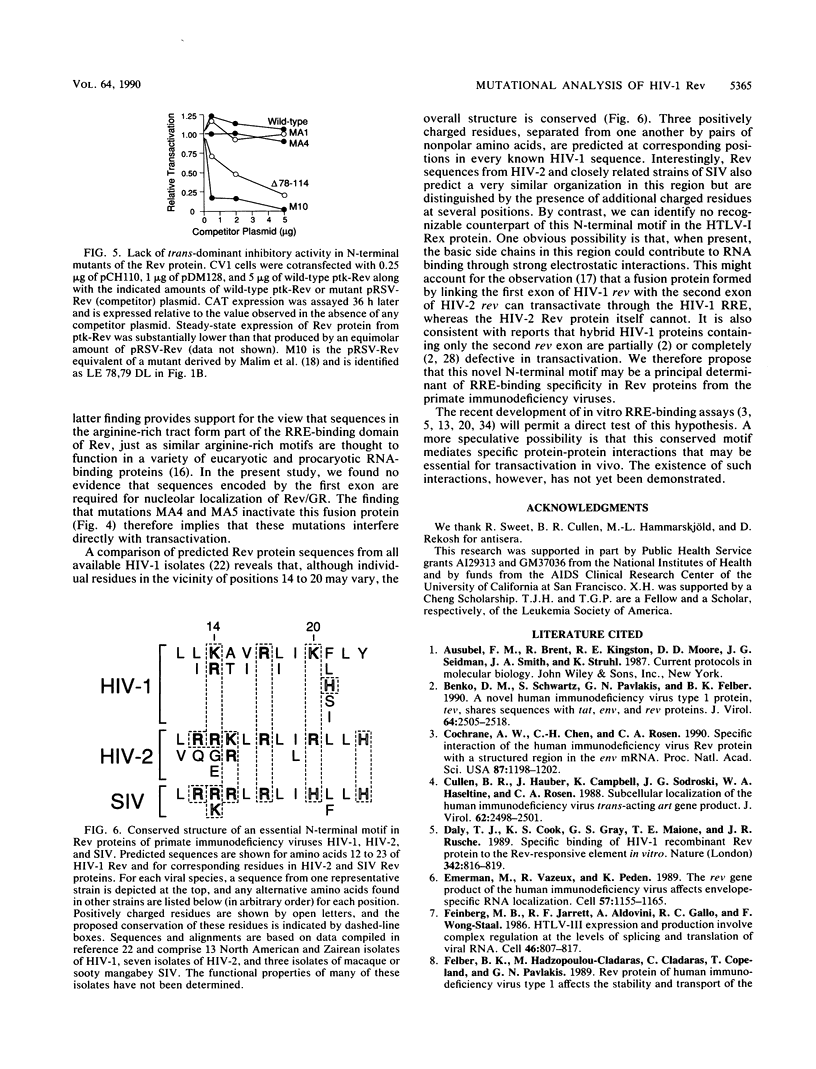
Abstract
The expression of certain mRNAs from human immunodeficiency virus type 1 (HIV-1) is controlled by the viral transactivator Rev, a nucleolar protein that binds a cis-acting element in these mRNAs. Rev is encoded by two viral exons that specify amino acids 1 to 26 and 27 to 116, respectively. Earlier studies have mapped essential regions of the protein that are encoded in the second exon. By further mutational analysis of Rev, we have now identified a novel locus encoded by the first exon that also is essential for transactivation in vivo. Defined by mutations at residues 14 to 20, this locus coincides with a cluster of positively charged and nonpolar amino acids that is conserved in Rev proteins of all known primate immunodeficiency viruses. Rev proteins that contained mutations at this site were defective in both nuclear localization and transactivation and did not function as trans-dominant inhibitors of wild-type Rev. Fusion of these mutants to a heterologous nuclear protein complemented the defect in localization but did not restore biological activity. Our findings suggest that this N-terminal locus may play a direct role in transactivation, perhaps contributing to essential protein-protein interactions or forming part of the RNA-binding domain of Rev.
Full text
PDF

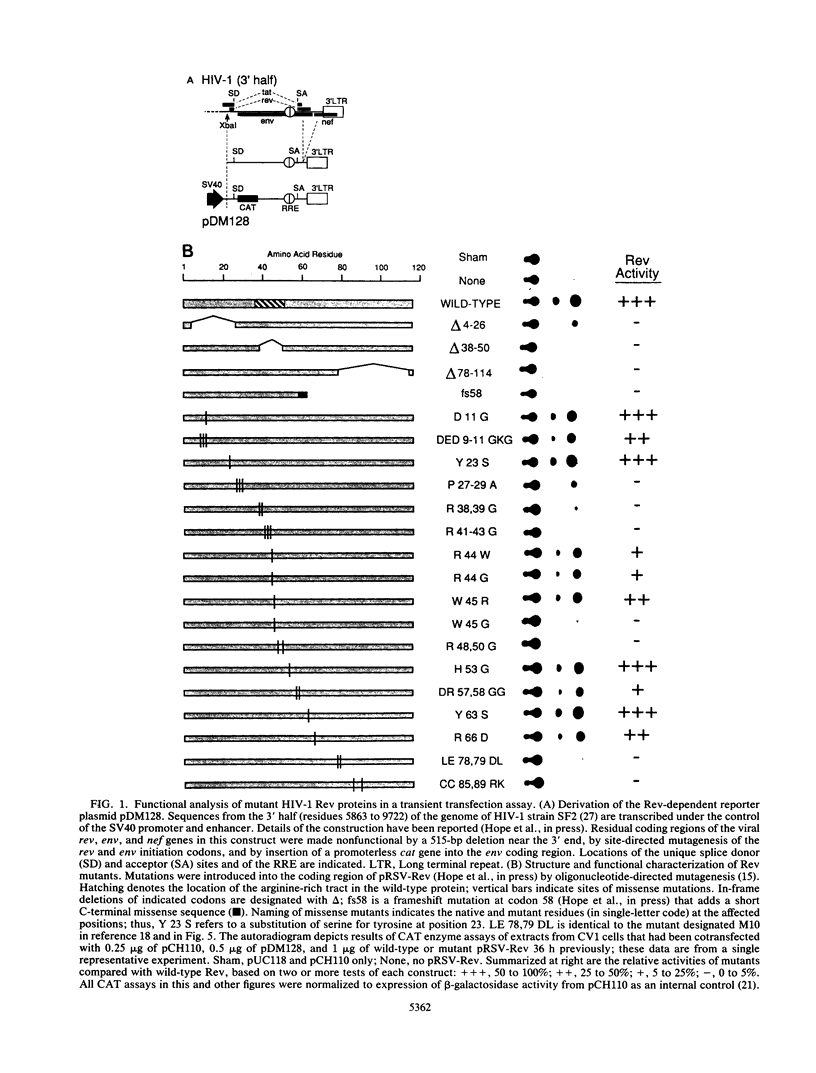
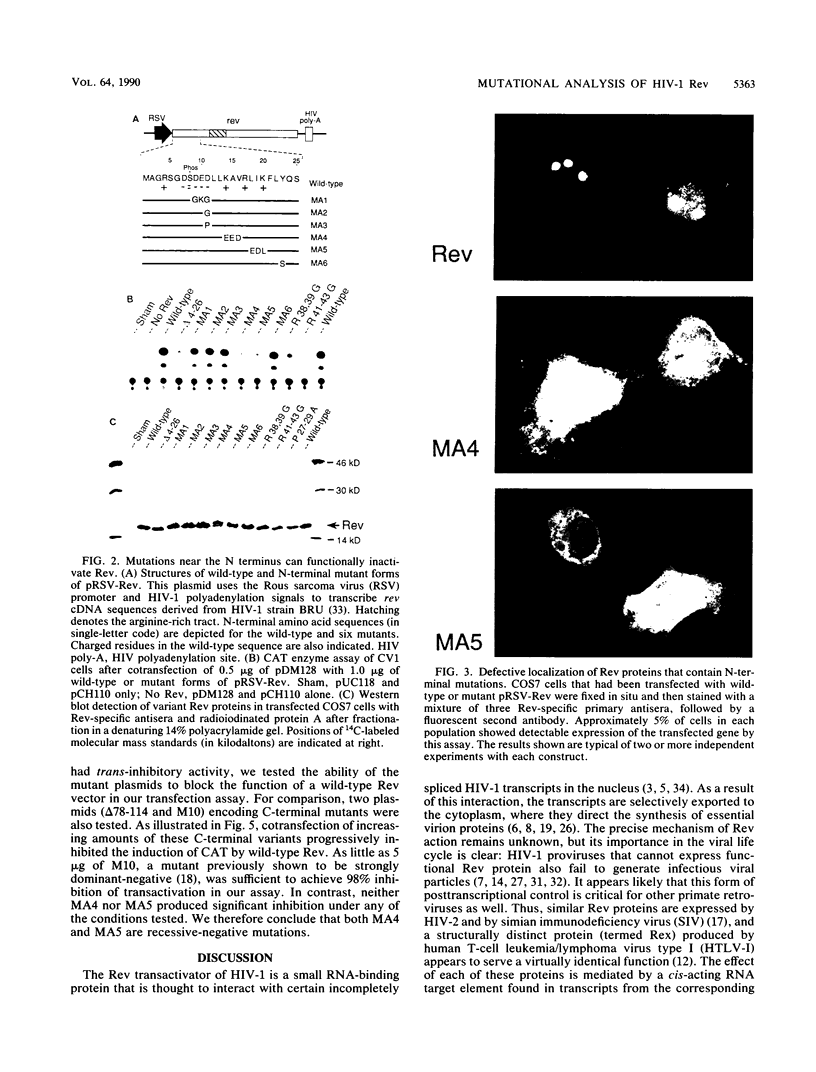

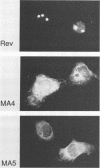
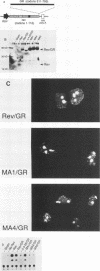
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko D. M., Schwartz S., Pavlakis G. N., Felber B. K. A novel human immunodeficiency virus type 1 protein, tev, shares sequences with tat, env, and rev proteins. J Virol. 1990 Jun;64(6):2505–2518. doi: 10.1128/jvi.64.6.2505-2518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane A. W., Chen C. H., Rosen C. A. Specific interaction of the human immunodeficiency virus Rev protein with a structured region in the env mRNA. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1198–1202. doi: 10.1073/pnas.87.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Hauber J., Campbell K., Sodroski J. G., Haseltine W. A., Rosen C. A. Subcellular localization of the human immunodeficiency virus trans-acting art gene product. J Virol. 1988 Jul;62(7):2498–2501. doi: 10.1128/jvi.62.7.2498-2501.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly T. J., Cook K. S., Gray G. S., Maione T. E., Rusche J. R. Specific binding of HIV-1 recombinant Rev protein to the Rev-responsive element in vitro. Nature. 1989 Dec 14;342(6251):816–819. doi: 10.1038/342816a0. [DOI] [PubMed] [Google Scholar]
- Emerman M., Vazeux R., Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989 Jun 30;57(7):1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Heimer J., Hammarskjöld B., Sangwan I., Albert L., Rekosh D. Regulation of human immunodeficiency virus env expression by the rev gene product. J Virol. 1989 May;63(5):1959–1966. doi: 10.1128/jvi.63.5.1959-1966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly S. M., Rimsky L. T., Malim M. H., Kim J. H., Hauber J., Duc Dodon M., Le S. Y., Maizel J. V., Cullen B. R., Greene W. C. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989 Oct;3(10):1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- Heaphy S., Dingwall C., Ernberg I., Gait M. J., Green S. M., Karn J., Lowe A. D., Singh M., Skinner M. A. HIV-1 regulator of virion expression (Rev) protein binds to an RNA stem-loop structure located within the Rev response element region. Cell. 1990 Feb 23;60(4):685–693. doi: 10.1016/0092-8674(90)90671-z. [DOI] [PubMed] [Google Scholar]
- Knight D. M., Flomerfelt F. A., Ghrayeb J. Expression of the art/trs protein of HIV and study of its role in viral envelope synthesis. Science. 1987 May 15;236(4803):837–840. doi: 10.1126/science.3033827. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazinski D., Grzadzielska E., Das A. Sequence-specific recognition of RNA hairpins by bacteriophage antiterminators requires a conserved arginine-rich motif. Cell. 1989 Oct 6;59(1):207–218. doi: 10.1016/0092-8674(89)90882-9. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Fenrick R., Le S. Y., Maizel J. V., Cullen B. R. Functional comparison of the Rev trans-activators encoded by different primate immunodeficiency virus species. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8222–8226. doi: 10.1073/pnas.86.21.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Hauber J., Cullen B. R. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989 Jul 14;58(1):205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Tiley L. S., McCarn D. F., Rusche J. R., Hauber J., Cullen B. R. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990 Feb 23;60(4):675–683. doi: 10.1016/0092-8674(90)90670-a. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Perkins A., Cochrane A. W., Ruben S. M., Rosen C. A. Structural and functional characterization of the human immunodeficiency virus rev protein. J Acquir Immune Defic Syndr. 1989;2(3):256–263. [PubMed] [Google Scholar]
- Rimsky L., Dodon M. D., Dixon E. P., Greene W. C. Trans-dominant inactivation of HTLV-I and HIV-1 gene expression by mutation of the HTLV-I Rex transactivator. Nature. 1989 Oct 5;341(6241):453–456. doi: 10.1038/341453a0. [DOI] [PubMed] [Google Scholar]
- Rimsky L., Hauber J., Dukovich M., Malim M. H., Langlois A., Cullen B. R., Greene W. C. Functional replacement of the HIV-1 rev protein by the HTLV-1 rex protein. Nature. 1988 Oct 20;335(6192):738–740. doi: 10.1038/335738a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Terwilliger E., Dayton A., Sodroski J. G., Haseltine W. A. Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2071–2075. doi: 10.1073/pnas.85.7.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie M. R., Benter T., Wong-Staal F. Site-directed mutagenesis of two trans-regulatory genes (tat-III,trs) of HIV-1. Science. 1988 Feb 19;239(4842):910–913. doi: 10.1126/science.3277284. [DOI] [PubMed] [Google Scholar]
- Salfeld J., Göttlinger H. G., Sia R. A., Park R. E., Sodroski J. G., Haseltine W. A. A tripartite HIV-1 tat-env-rev fusion protein. EMBO J. 1990 Mar;9(3):965–970. doi: 10.1002/j.1460-2075.1990.tb08195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Terwilliger E., Burghoff R., Sia R., Sodroski J., Haseltine W., Rosen C. The art gene product of human immunodeficiency virus is required for replication. J Virol. 1988 Feb;62(2):655–658. doi: 10.1128/jvi.62.2.655-658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Zapp M. L., Green M. R. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature. 1989 Dec 7;342(6250):714–716. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]