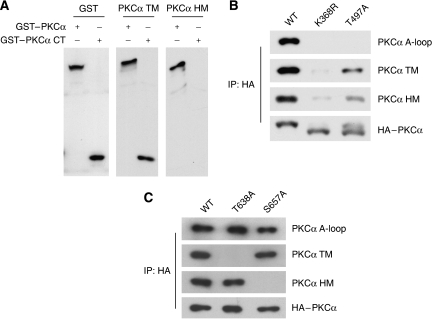
Figure 3.
Relationship among different PKCα phosphorylation sites. (A) Kinase domain is not required for PKCα TM phosphorylation. HEK293T cells were transfected with indicated GST–PKCα full length or C-terminal fragment (CT, a.a. 603–672). Cell lysates were probed with antibody for GST, TM and HM as indicated. (B) Phosphorylation status of PKCα mutants. Transfected HA-tagged PKCα (in HEK293T) were immunoprecipitated with HA-antibody. Phosphorylation was determined by immunoblotting with indicated antibodies. K368R and T497A denote PKCα kinase inactive and A-loop mutant, respectively. (C) Relationship between PKCα TM and HM phosphorylation. HEK293T cells were transfected with PKCα wild type (WT), TM mutant (T638A) and HM mutant (S657A). Phosphorylation of each PKCα construct was determined.