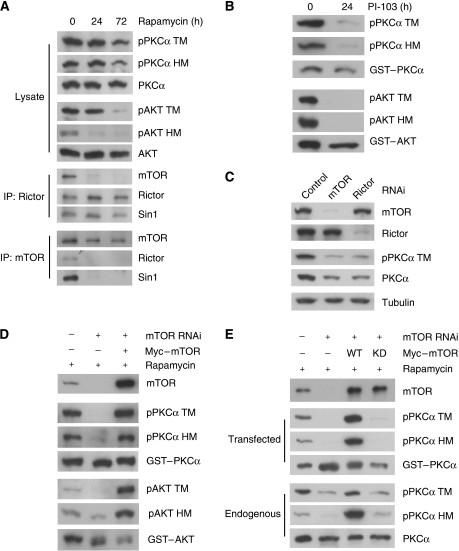
Figure 5.
mTORC2 integrity and mTOR kinase activity are required for PKCα and Akt TM phosphorylation. (A) Prolonged treatment of rapamycin decreases both TM and HM phosphorylation in PKCα and Akt. HepG2 cells were treated with 100 nM rapamycin for indicated times. Total cell lysates were probed with PKC and Akt antibodies as indicated. Rictor or mTOR immunoprecipitates were analysed by immunoblotting with antibodies against TORC2 components. (B) The PI3K/mTOR inhibitor P103 inhibits phosphorylation of transfected PKCα and Akt. HEK293T cells were transfected with GST–PKCα and GST–Akt in the presence or absence of 10 μM PI-103. Immunoblottings were performed using indicated antibodies. (C) mTOR and Rictor are required for maintaining both phosphorylation and protein level of PKCα. HeLa cells stably expressing control, mTOR shRNA or Rictor shRNA were transfected with control, mTOR or Rictor siRNA oligos, respectively. (D) Knockdown of mTOR combined with prolonged rapamycin treatment abolishes TM phosphorylation. HeLa cells with mTOR knockdown using shRNA-containing lentivirus were pretreated with 100 nM rapamycin for 24 h. mTOR siRNA oligos together with GST–PKCα, GST–Akt, and siRNA-resistant Myc-mTOR were transfected as indicated under rapamycin treatment. (E) Kinase activity of mTOR is required for PKCα TM phosphorylation. Experiments were similar to Figure 5D except that the siRNA-resistant kinase dead (KD) mTOR was included. Protein levels and phosphorylation of both the transfected GST–PKCα and the endogenous PKCα were determined.