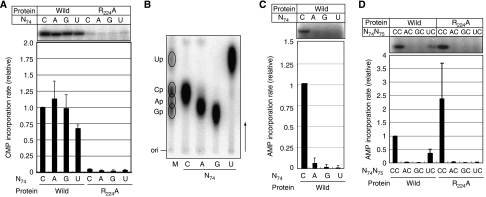
Figure 2.
CTP and ATP incorporation into mutant mini-helix variants. (A) CMP incorporation into mini-D73N74 in the presence of α-32P CTP and unlabelled ATP by the wild-type AFCCA (left part) or the R224A mutant AFCCA (right part). The upper panel shows the autoradiograph of the gel, and the lower graph shows the quantification of the relative initial velocity of CMP incorporation. The absolute CMP incorporation rate was estimated as 1.95 pmol min−1 μg−1, and it was defined as 1.0. (B) Neighbouring nucleotide analysis of 32P-labelled products in (A) by thin-layer chromatography. M indicates the RNase T2 hydrolysate of a uniformly α-32P UTP-labelled E. coli tRNAGlu transcript, as a marker. C, A, G, and U indicate RNase T2 hydrolysates of 32P-labelled products in the gels in the left part of (A). (C) AMP incorporation into mini-D73N74 in the presence of unlabelled CTP and α-32P ATP by the wild-type AFCCA. The upper panel shows the autoradiograph of the gel, and the lower graph shows the quantification of the relative initial velocity of AMP incorporation. The absolute AMP incorporation rate was estimated as 0.256 pmol min−1 μg−1, and it was defined as 1.0. (D) AMP incorporation into mini-D73N74C75 by the wild-type AFCCA (left part) and the R224A mutant AFCCA (right part). The presentation is the same as in (A). The absolute AMP incorporation rate into mini-D73C74C75 was estimated as 3.34 pmol min−1 μg−1, and it was defined as 1.0. The bars on the graphs indicate the standard deviations of more than three independent experiments.