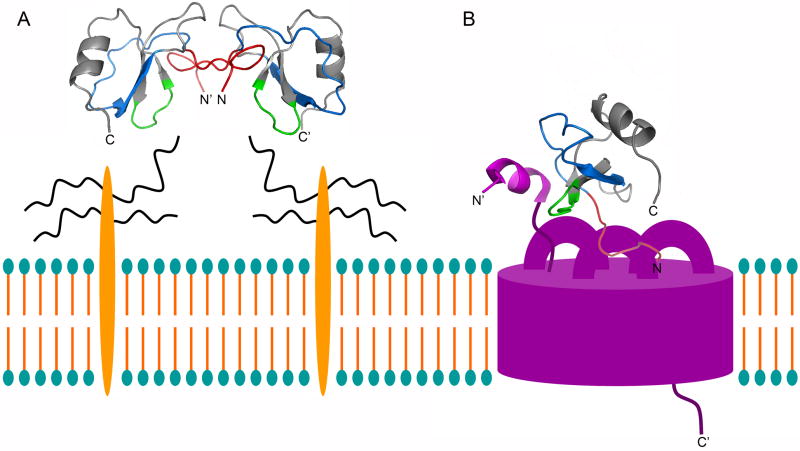
Figure 1.
Schematic representation of RANTES binding to GAGs and CCR5 on the cell surface. A, Ribbon side view representation of the RANTES dimer (PDB entry 1HRJ) and its interaction with cell surface glycosaminoglycans (the protein core is represented in orange; the carbohydrate chains in black). The N-terminus (aa 1-10) is colored in red; the N-loop/β1-strand (aa 11-29) in blue; the GAG-binding 40’s loop (aa 43-48) in green. N and C denote the N- and C-terminus, respectively (N′ and C′, the termini of the second RANTES monomer within the dimer). B, Side view of the RANTES monomer (color code as in A) in a proposed complex with CCR5 (pink). The RANTES monomer is shown with a 45° anti-clockwise rotation compared to A, and is slightly tilted towards the observer. The α-helix conformation adopted by the CCR5 N-terminal peptide, spanning residues 7-15, corresponds to that illustrated in PDB entry 2RLL. As previously suggested [48], the RANTES N-terminus is likely to be embedded within transmembrane CCR5 helices, while the extracellular CCR5 loops, particularly ECL2, should surround RANTES. N and C denote RANTES N- and C-terminus, respectively (N′ and C′, denote the CCR5 termini). In both A and B, RANTES orientation towards GAGs, CCR5 and the cell membrane have been chosen arbitrarily.