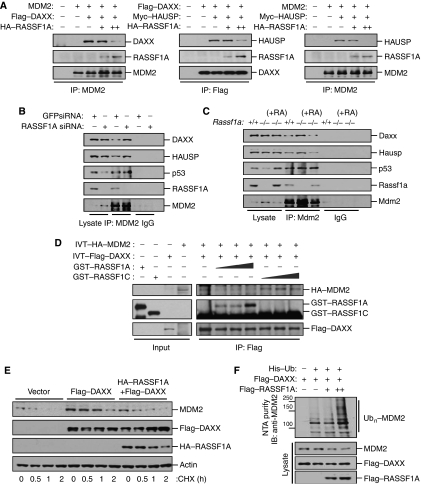
Figure 6.
RASSF1A increases MDM2 self-ubiquitination by disrupting MDM2–DAXX–HAUSP interactions. (A) Mdm2−/−p53−/− MEFs transfected with MDM2 and Flag–DAXX or Myc–HAUSP and increasing amounts of HA–RASSF1A were treated with MG132 for 6 h. Cell lysates were then immunoprecipitated (IP) with anti-MDM2 or anti-Flag antibodies, and the resulting precipitates were analysed by immunoblotting. (B) U2OS cells transfected with GFP siRNA or RASSF1A siRNA were immunoprecipitated (IP) with an anti-MDM2 antibody and the resulting precipitates were analysed by immunoblotting. (C) Rassf1a+/+, Rassf1a−/−, and Rassf1a−/− MEFs complemented with pcDNA-RASSF1A were immunoprecipitated (IP) with anti-Mdm2 and the resulting precipitates were analysed by immunoblotting. (D) GST–RASSF1A (70, 140, and 210 ng) or GST–RASSF1C (70, 140, and 210 ng) were added to a mixture of in vitro-translated (IVT) HA–MDM2 and Flag–DAXX and incubated for 2 h at room temperature, followed by immunoprecipitation (IP) with an anti-Flag antibody and immunoblotting. (E) U2OS cells transfected with Flag–DAXX with or without HA–RASSF1A were incubated for the indicated times with cycloheximide (50 μg/ml) and analysed by immunoblotting. (F) U2OS cells were transfected with a combination of Flag–DAXX and Flag–RASSF1A in the presence of a His6-tagged ubiquitin expression plasmid. Cells were treated with MG132 for 6 h prior to harvesting. The His6-purified fractions were analysed for ubiquitinated MDM2.