Abstract
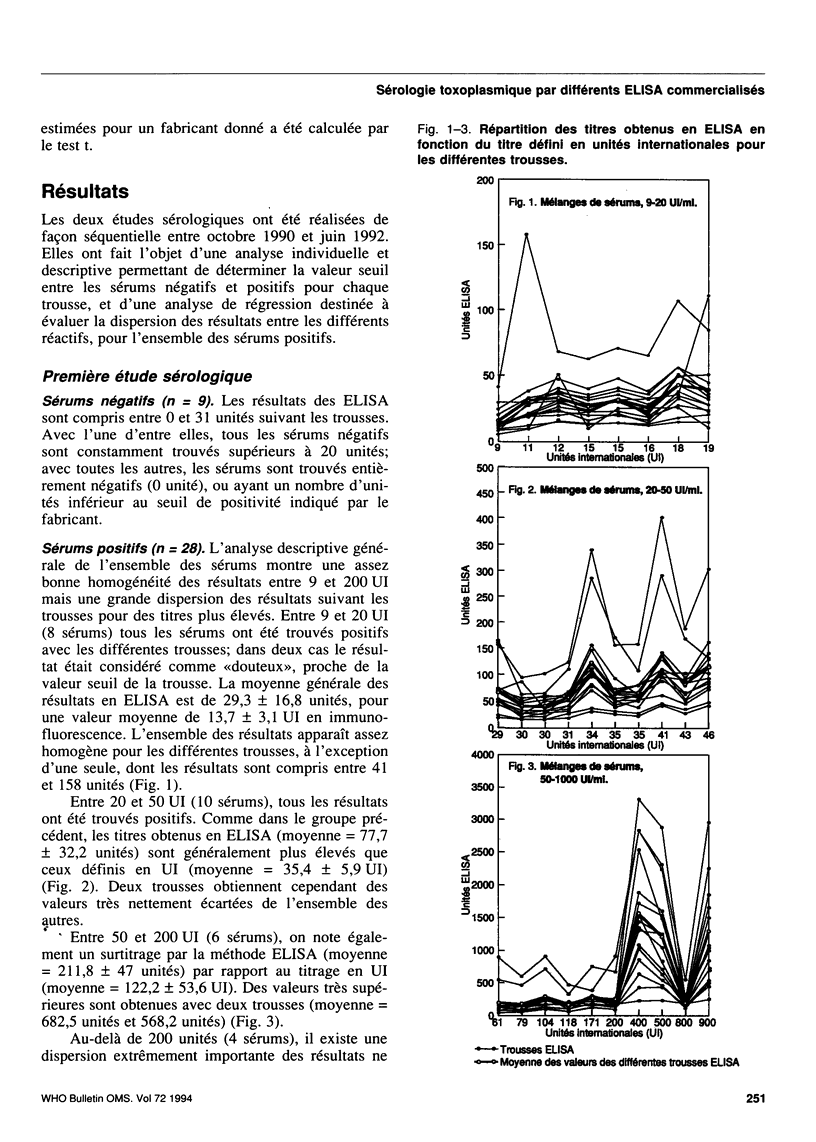
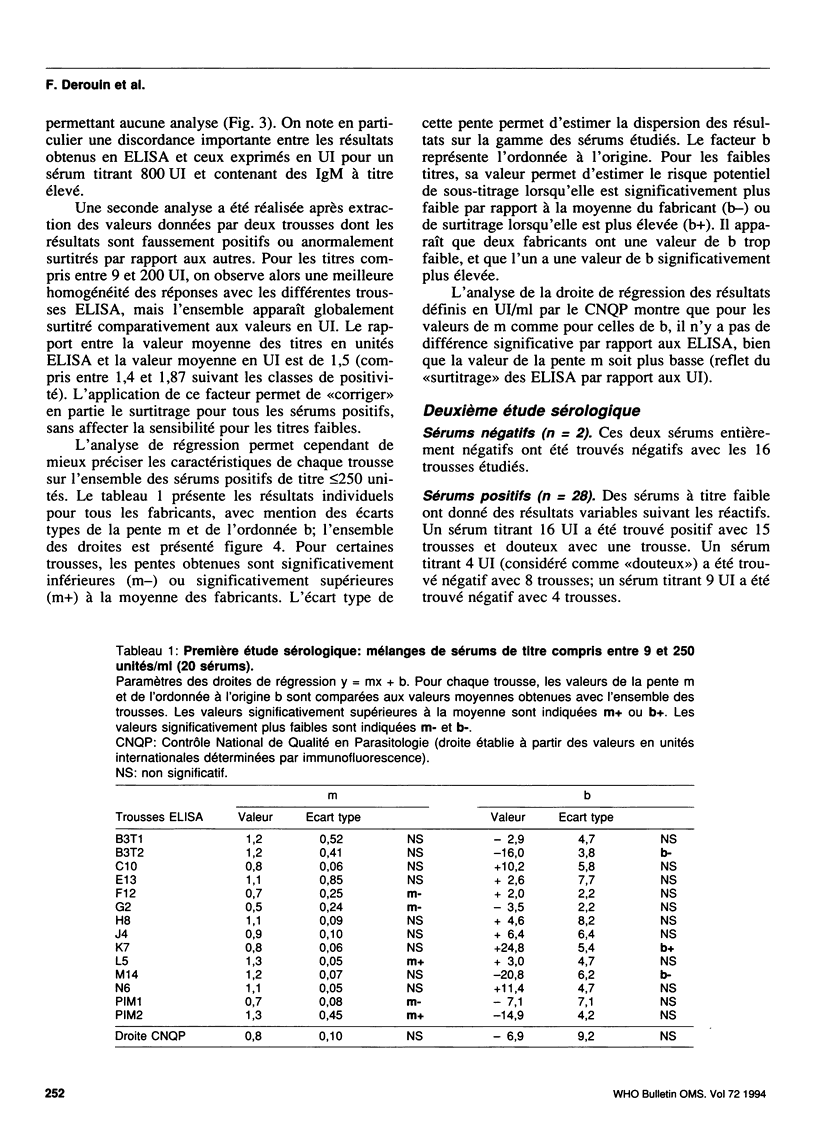
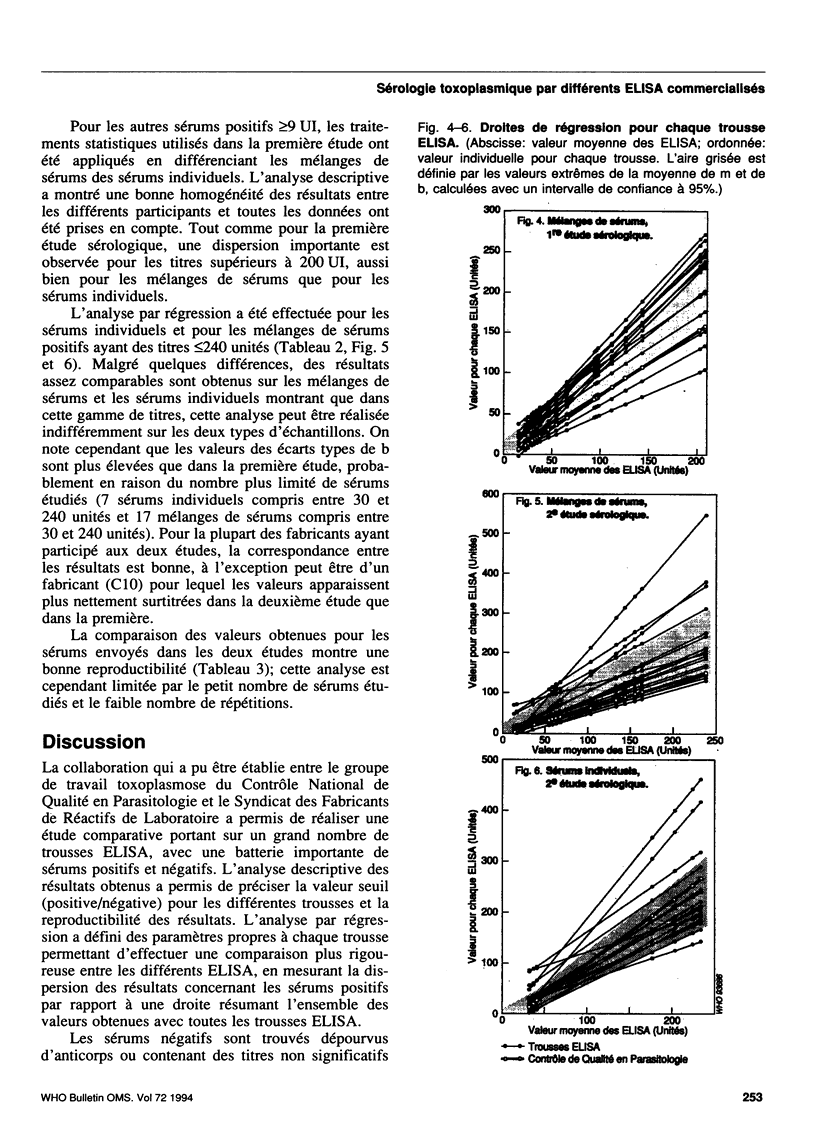
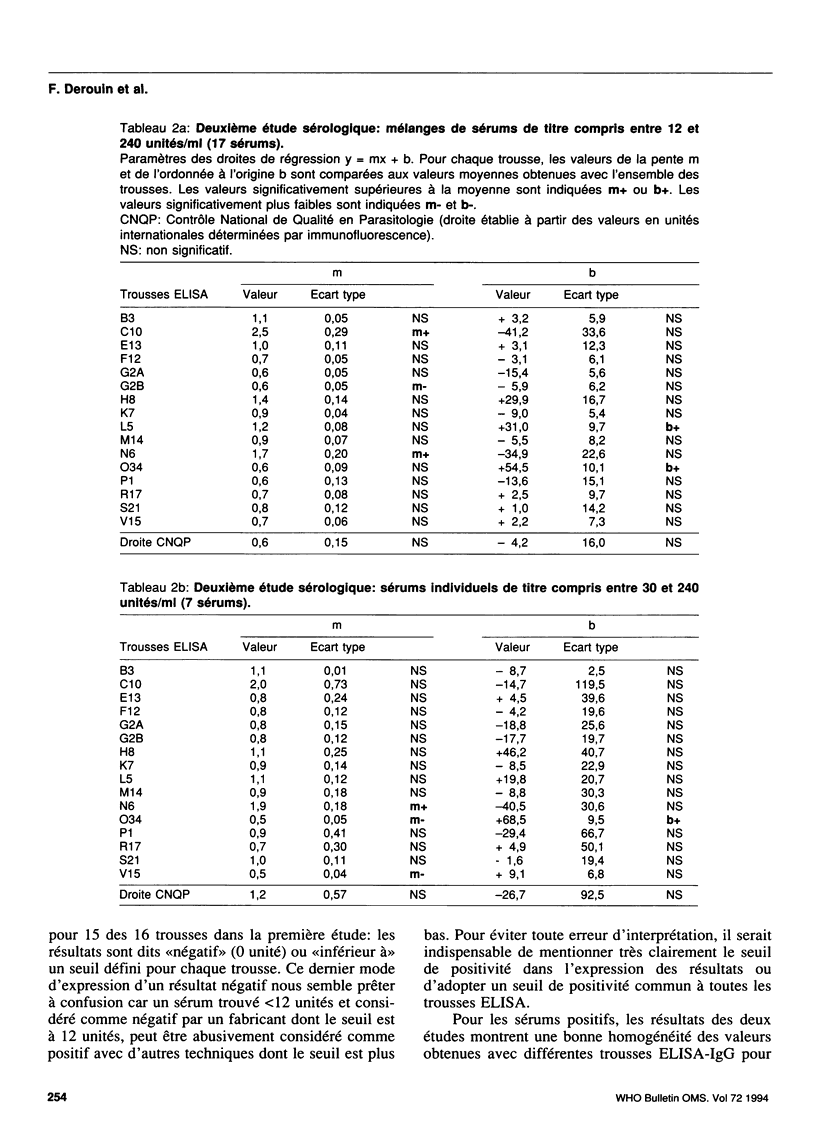
A collaborative study conducted by the French National Agency for Quality Control in Parasitology (CNQP) and various manufacturers of ELISA kits, represented by the Association of Laboratory Reagent Manufacturers (SFRL) compared the toxoplasmosis IgG antibody titres obtained with different ELISA-IgG kits and determined the relationships between the titres obtained by these techniques and the titre defined in international units (IU). Fifty-one serum samples with toxoplasmosis antibody titres ranging from 0 to 900 IU were tested in two successive studies with 16 ELISA-IgG kits. For the negative sera, false-positive reactions were observed with one kit. For the positive sera, the titres observed in ELISA were generally higher than those expressed in IU. Above 250 IU, the very wide variability of the titres found with the different ELISA kits renders any comparative analysis impossible. For titres below 250 IU, the results are sufficiently homogeneous to permit the use of regression analysis to study how the results for each ELISA kit compare with the mean results for the other kits. The slope of the line of regression shows a tendency to over-titration or under-titration compared with the results of the other manufacturers; the ordinate at the origin reflects the positivity threshold of the reaction and can be used to assess the risk of a lack of sensitivity (high threshold) or of specificity (threshold too low). On the whole, the trends revealed for a given manufacturer are constant from one study to the other. Within this range of titres, regression analysis also reveals the general tendency of ELISA kits to overestimate the titres by comparison with immunofluorescence.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlier Y., Bout D., Dessaint J. P., Capron A., Van Knapen F., Ruitenberg E. J., Bergquist R., Huldt G. Evaluation of the enzyme-linked immunosorbent assay (ELISA) and other serological tests for the diagnosis of toxoplasmosis. Bull World Health Organ. 1980;58(1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Thulliez P., Garin Y. J. Intérêt et limites de la sérologie de toxoplasmose chez les sujets VIH+. Pathol Biol (Paris) 1991 Apr;39(4):255–259. [PubMed] [Google Scholar]
- Hedman K., Lappalainen M., Seppäiä I., Mäkelä O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989 Apr;159(4):736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- Joynson D. H., Payne R. A., Balfour A. H., Prestage E. S., Fleck D. G., Chessum B. S. Five commercial enzyme linked immunosorbent assay kits for toxoplasma specific IgM antibody. J Clin Pathol. 1989 Jun;42(6):653–657. doi: 10.1136/jcp.42.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niel G., Desmonts G., Gentilini M. Immunofluorescence quantitative et diagnostic sérologique de la toxoplasmose: introduction des unités internationales dans l'expression des positivés. Pathol Biol (Paris) 1973 Feb;21(2):157–161. [PubMed] [Google Scholar]
- Potasman I., Araujo F. G., Desmonts G., Remington J. S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986 Oct;154(4):650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- Wilson M., Ware D. A., Walls K. W. Evaluation of commercial serodiagnostic kits for toxoplasmosis. J Clin Microbiol. 1987 Dec;25(12):2262–2265. doi: 10.1128/jcm.25.12.2262-2265.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


