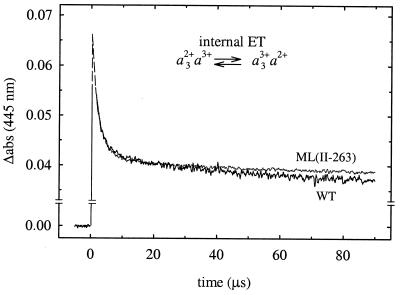
Figure 3.
Electron equilibration between hemes a and a3 after flash photolysis of CO (at t = 0) from the wild-type and ML(II-263) mutant mixed-valence-CO enzymes. The rapid increase following immediately after the flash is associated with dissociation of CO. The decrease in absorbance with a time constant of about 3 μs is associated with fractional electron transfer from heme a3 to heme a. The slower absorbance decrease after the 3-μs phase is associated with CO recombination. The absorbances have been extrapolated (dashed lines) to t = 0 from the fitted exponentials. The traces shown are the average of 15 individual traces. Experimental conditions: 22°C, 0.1 M Hepes, pH 7.4/0.1% β-d-dodecyl maltoside/1.3 μM reacting enzyme (note that in the graph the traces have been scaled to 1 μM enzyme)/1 mM CO.