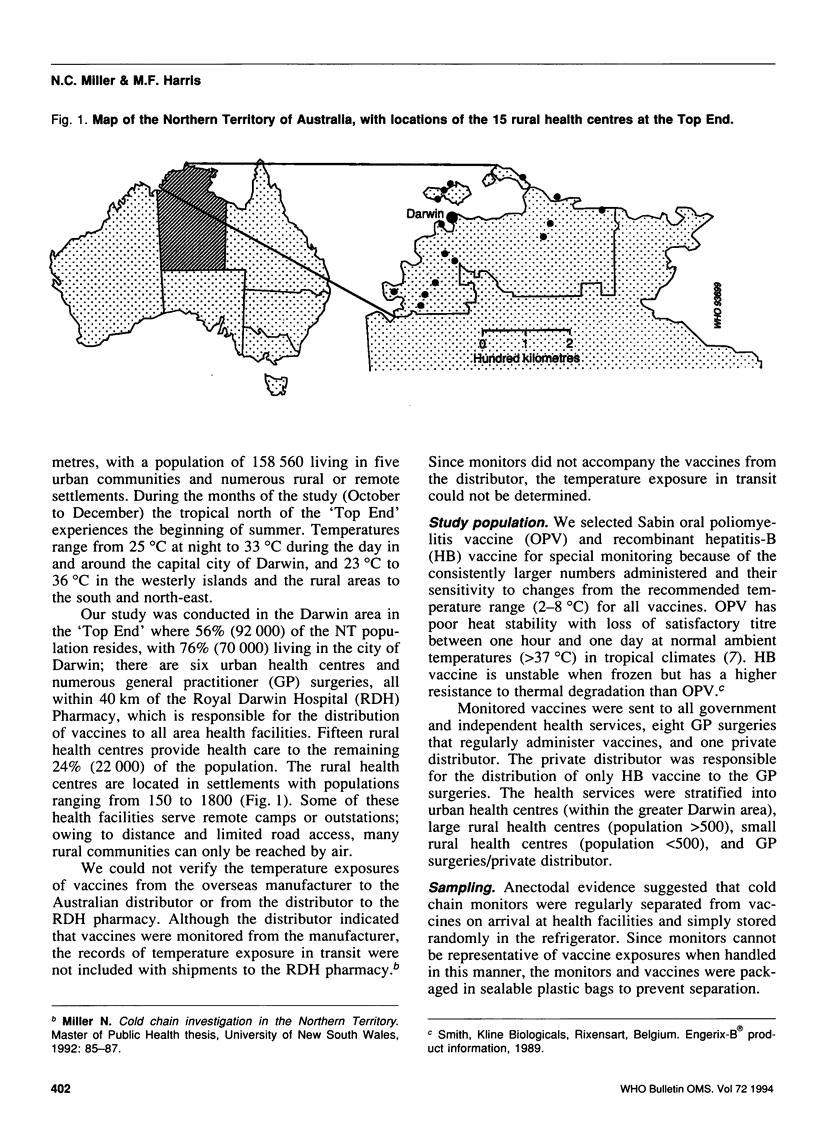
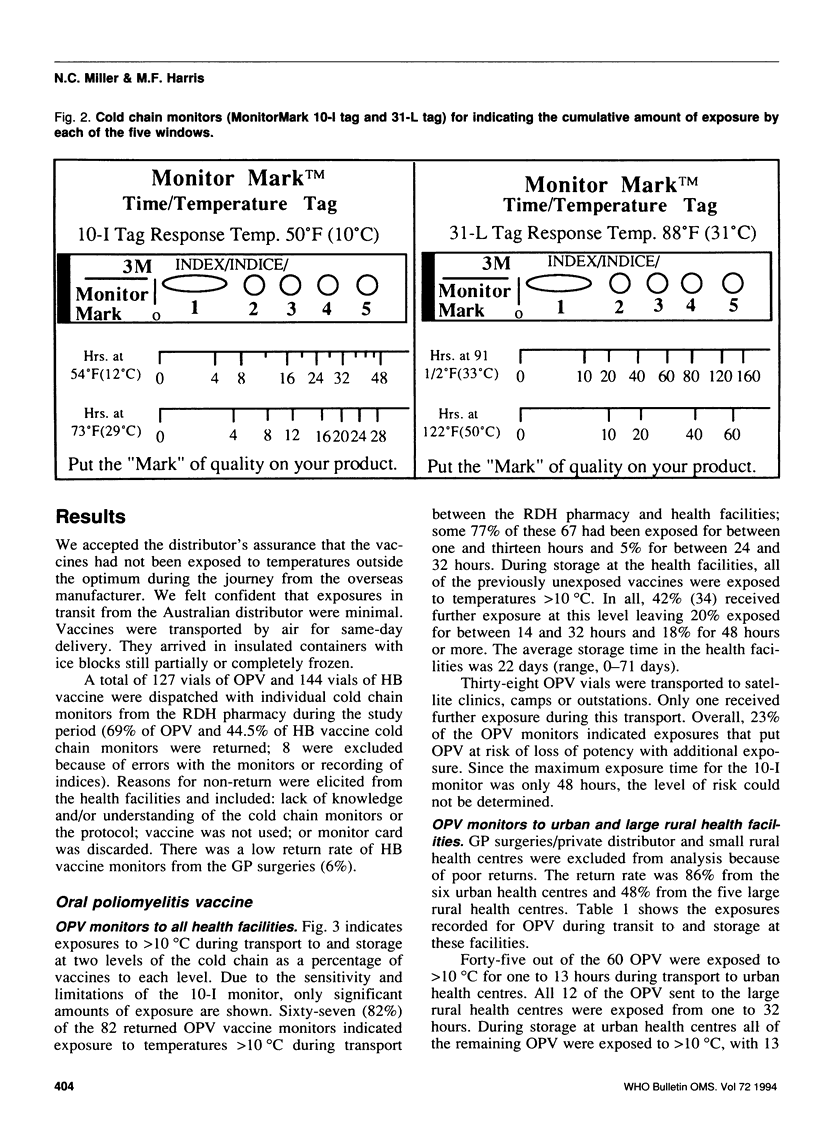
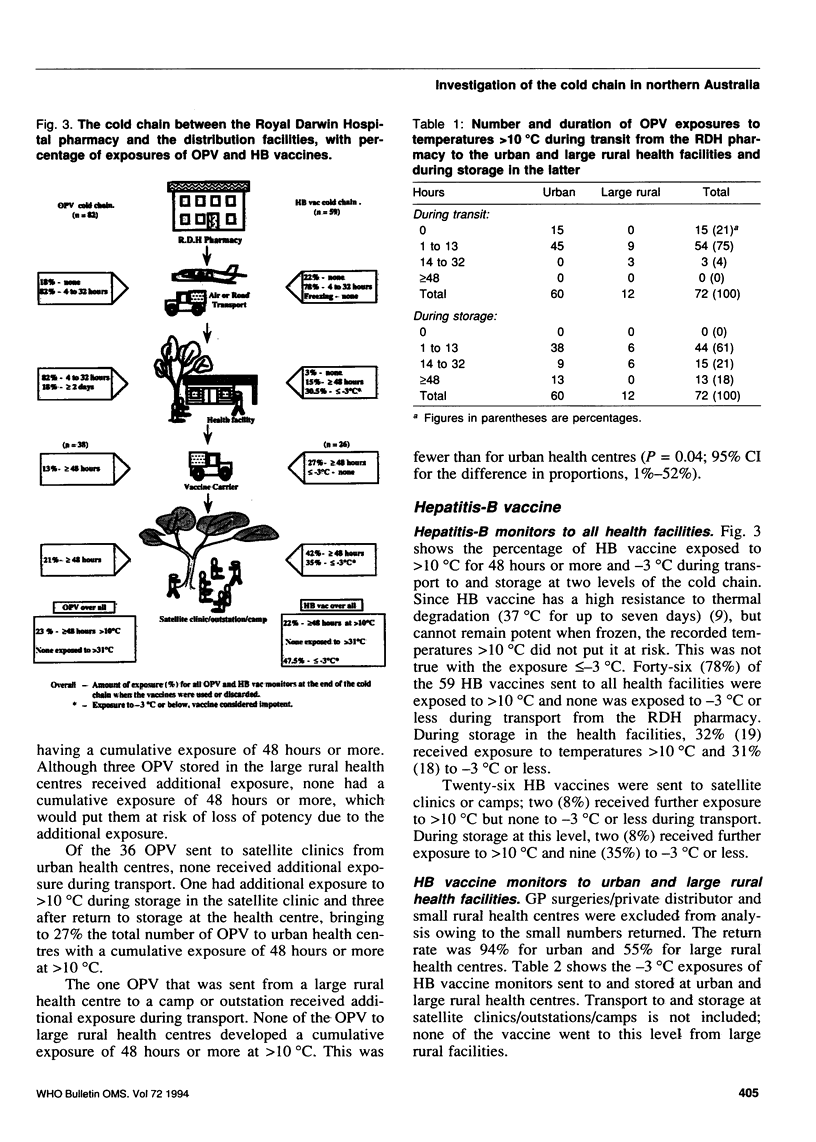
Abstract
Since vaccines may lose their potency if transported or stored outside the recommended temperature range (2-8 degrees C), we carried out a study in the Darwin area of the Northern Territory of Australia to determine the links in the cold chain, including the extent of vaccine monitoring, and whether the vaccines were being exposed to unsafe temperatures. Sabin oral poliomyelitis vaccine (OPV) and recombinant hepatitis-B (HB) vaccine were selected for special monitoring. A total of 127 vials of OPV and 144 vials of HB vaccine were dispatched during October, November and December 1990 to the government, independent health services and general practitioner surgeries which routinely administer these vaccines. We distributed the two vaccines with MonitorMark time/temperature and Coldside indicator tags attached to cards for recording the date, location and temperature exposures each time the vaccines were moved or used. A total of 65% of the OPV and 41% of the HB vaccine monitor cards were returned for analysis. The vaccines were transported and stored at one to four locations prior to being administered. Some 23% of tagged OPV was exposed for 48 hours or more to a temperature > 10 degrees C; 47.5% of tagged HB vaccines were exposed to -3 degrees C or less, the majority of them during storage in health facilities or clinics. Exposures were independent of distance from the distribution centre, mode of transport, or type of facility. Our results show that the vaccines were often exposed to temperatures outside the recommended range during transport and storage, putting them at risk of loss of potency.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrand J., Chapman J., Jeffs D. A., Jack I., Wenzel W. A., Bridges-Webb C., Levy M. H. Reported measles immunisation and serological immunity in children attending general practitioners. Aust J Public Health. 1991 Jun;15(2):101–106. doi: 10.1111/j.1753-6405.1991.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Hanna J. N. Poor response to hepatitis B vaccine administered to aboriginal infants in Central Australia. Med J Aust. 1987 May 4;146(9):504–505. doi: 10.5694/j.1326-5377.1987.tb120378.x. [DOI] [PubMed] [Google Scholar]
- Hunter S. Storage of vaccines in general practice. BMJ. 1989 Sep 9;299(6700):661–662. doi: 10.1136/bmj.299.6700.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugosi L., Battersby A. Transport and storage of vaccines in Hungary: the first cold chain monitor study in Europe. Bull World Health Organ. 1990;68(4):431–439. [PMC free article] [PubMed] [Google Scholar]
- The cold chain for vaccine conservation: recent improvements. WHO Chron. 1979 Oct;33(10):383–386. [PubMed] [Google Scholar]


