Abstract
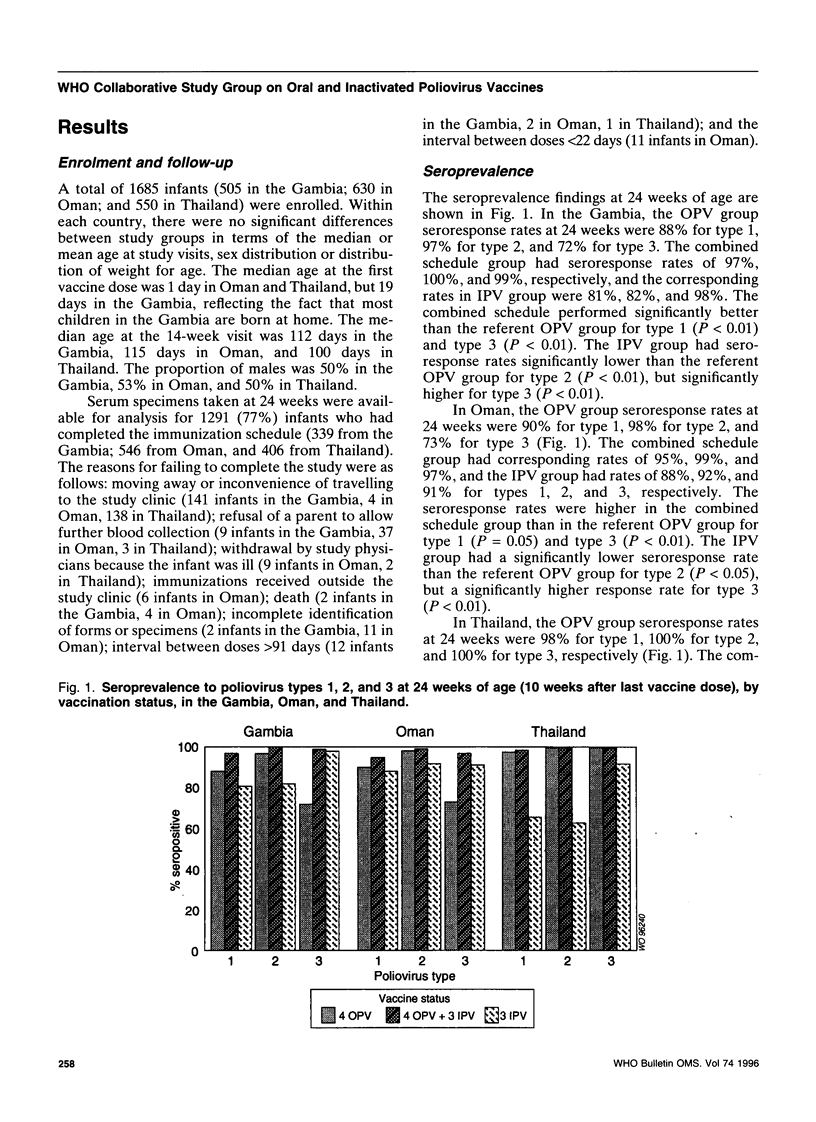
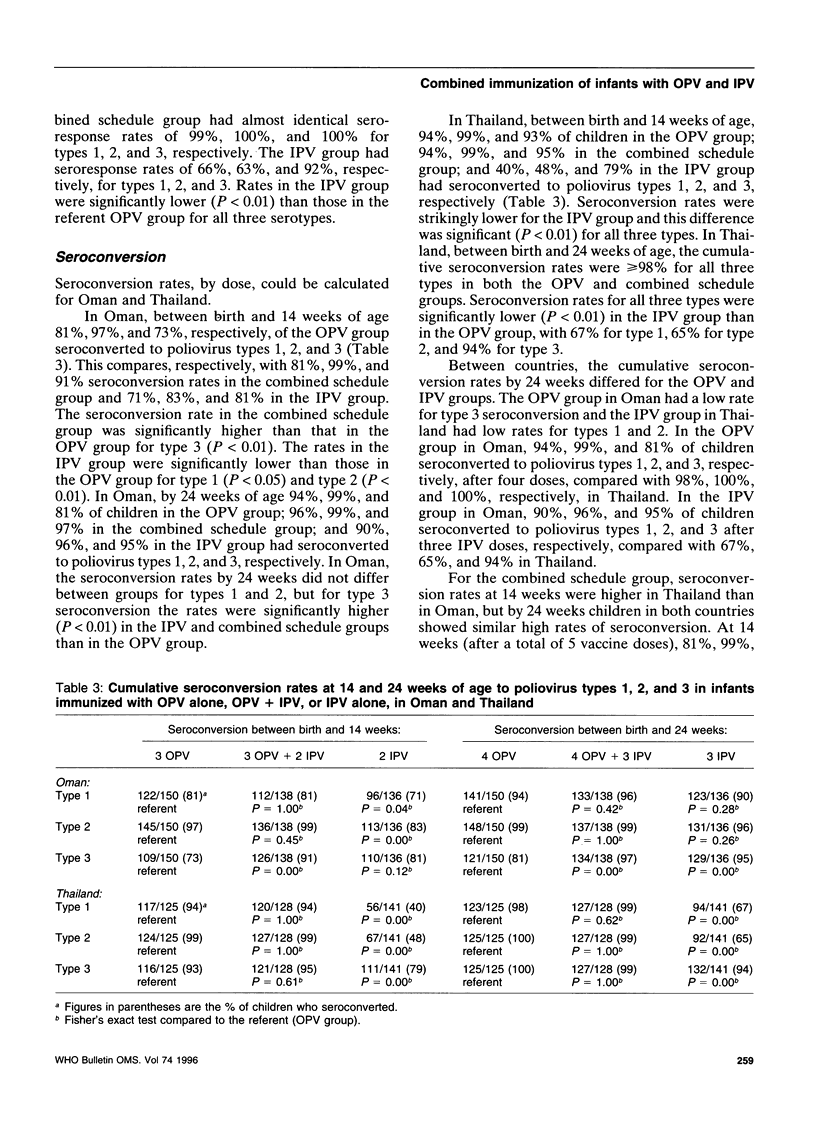
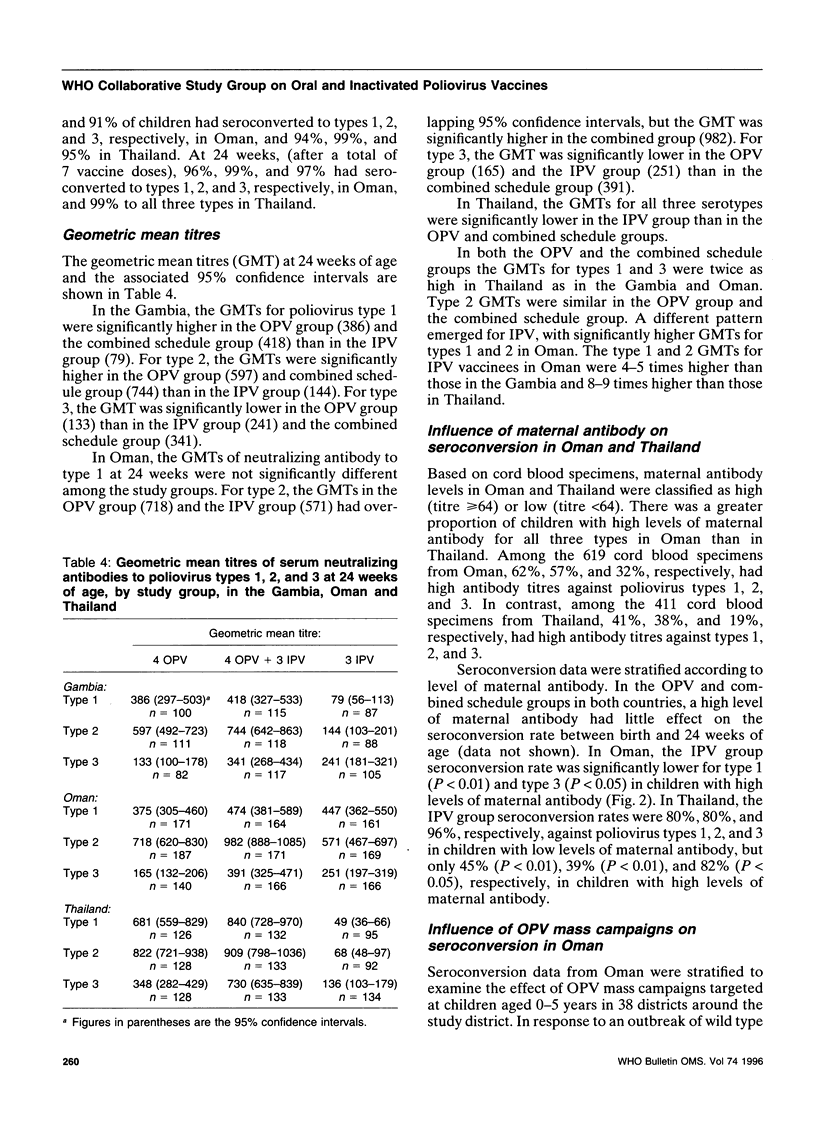
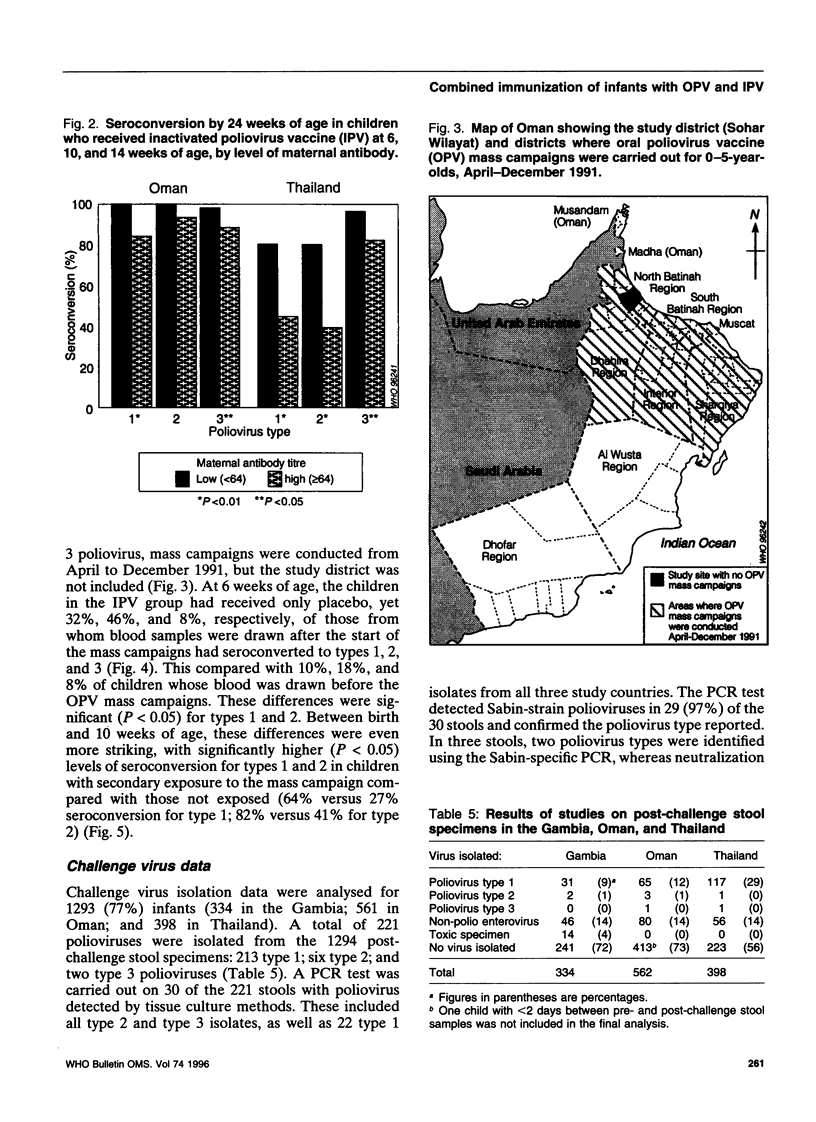
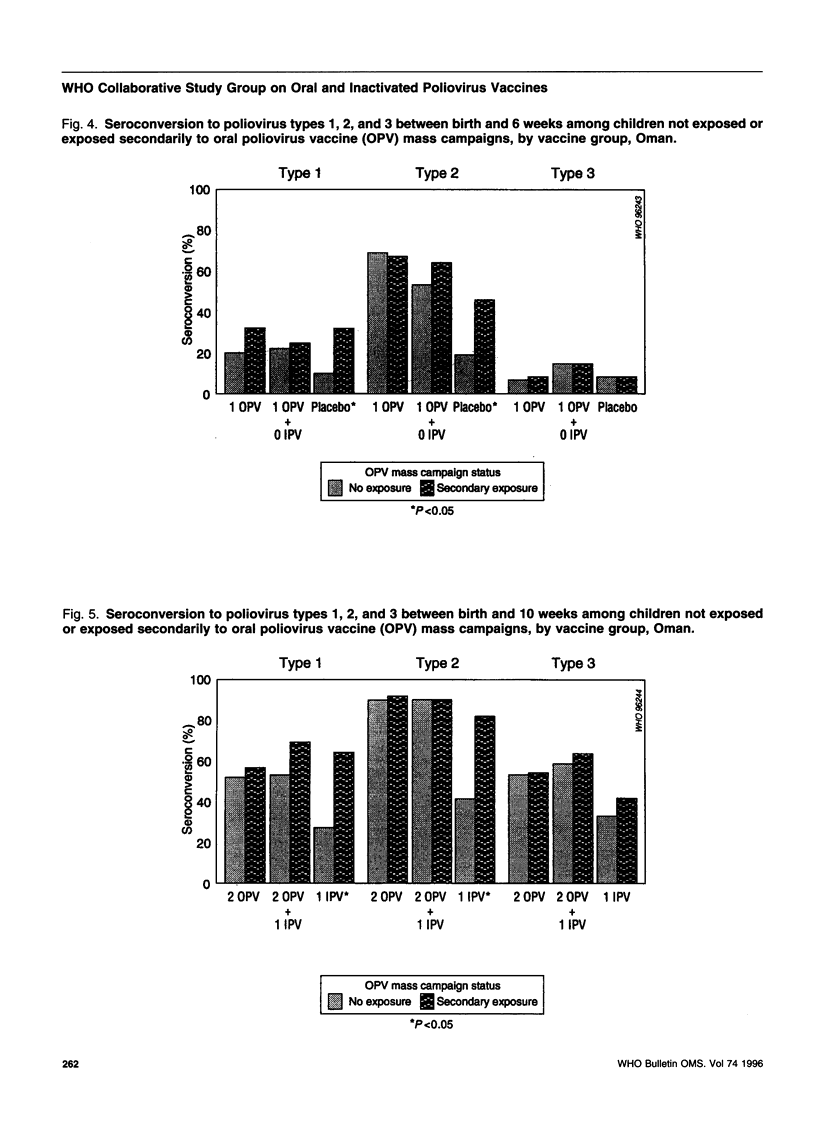
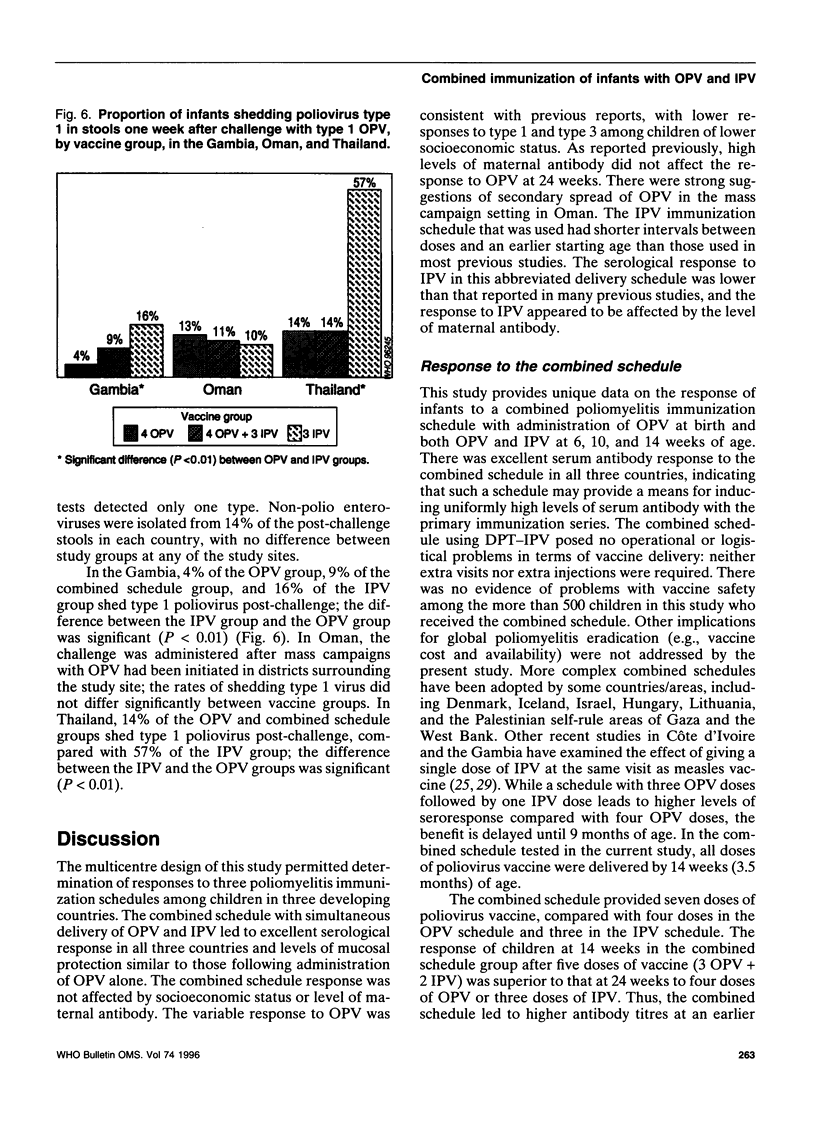
To assess an immunization schedule combining oral (OPV) and inactivated poliovirus vaccines (IPV), we conducted a clinical trial in the Gambia, Oman, and Thailand. Children were randomized to receive one of the following schedules: OPV at birth, 6, 10, and 14 weeks of age; OPV at birth followed by both OPV and IPV at 6, 10, and 14 weeks of age: or placebo at birth followed by IPV at 6, 10, and 14 weeks of age. A total of 1685 infants were enrolled; 24-week serum specimens were available for 1291 infants (77%). Across the study sites at 24 weeks of age, the proportion of seropositive children in the combined schedule group was 95-99% for type 1, 99-100% for type 2, and 97-100% for type 3. In the Gambia and Oman, the combined schedule performed significantly better than OPV for type 1 (95-97% versus 88-90%) and type 3 (97-99% versus 72-73%). In the Gambia and Oman, seroprevalences in the IPV group were lower for type 1 (significantly lower in the Gambia); significantly lower for type 2; and significantly higher for type 3, compared with the OPV group. In Thailand, the IPV group had significantly lower proportions of children who were seropositive for each of the three types, compared with the OPV group. The responses to OPV in the Gambia, Oman, and Thailand were consistent with previous studies from these countries. IPV given at 6, 10, and 14 weeks of age provided inadequate serological protection against poliovirus, especially type 1. The combined schedule provided the highest levels of serum antibody response, with mucosal immunity equivalent to that produced by OPV alone.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNKOPF H., MEDALIE J., YEKUTIEL M. Antibodies to poliomyelitis virus and socioeconomic factors influencing their frequency in children in Israel. Am J Trop Med Hyg. 1957 Jul;6(4):697–703. doi: 10.4269/ajtmh.1957.6.697. [DOI] [PubMed] [Google Scholar]
- Butler J. E., Satam M., Ekstrand J. Fluoride: an adjuvant for mucosal and systemic immunity. Immunol Lett. 1990 Dec;26(3):217–220. doi: 10.1016/0165-2478(90)90149-k. [DOI] [PubMed] [Google Scholar]
- Coursaget P., Relyveld E., Brizard A., Frenkiel M. P., Fritzell B., Teulières L., Bourdil C., Yvonnet B., Jeannée E., Guindo S. Simultaneous injection of hepatitis B vaccine with BCG and killed poliovirus vaccine. Vaccine. 1992;10(5):319–321. doi: 10.1016/0264-410x(92)90370-y. [DOI] [PubMed] [Google Scholar]
- Deming M. S., Jaiteh K. O., Otten M. W., Jr, Flagg E. W., Jallow M., Cham M., Brogan D., N'jie H. Epidemic poliomyelitis in The Gambia following the control of poliomyelitis as an endemic disease. II. Clinical efficacy of trivalent oral polio vaccine. Am J Epidemiol. 1992 Feb 15;135(4):393–408. doi: 10.1093/oxfordjournals.aje.a116300. [DOI] [PubMed] [Google Scholar]
- Faden H., Modlin J. F., Thoms M. L., McBean A. M., Ferdon M. B., Ogra P. L. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis. 1990 Dec;162(6):1291–1297. doi: 10.1093/infdis/162.6.1291. [DOI] [PubMed] [Google Scholar]
- Giammanco G., Li Volti S., Mauro L., Bilancia G. G., Salemi I., Barone P., Musumeci S. Immune response to simultaneous administration of a recombinant DNA hepatitis B vaccine and multiple compulsory vaccines in infancy. Vaccine. 1991 Oct;9(10):747–750. doi: 10.1016/0264-410x(91)90291-d. [DOI] [PubMed] [Google Scholar]
- Goldblum N., Gerichter C. B., Tulchinsky T. H., Melnick J. L. Poliomyelitis control in Israel, the West Bank and Gaza Strip: changing strategies with the goal of eradication in an endemic area. Bull World Health Organ. 1994;72(5):783–796. [PMC free article] [PubMed] [Google Scholar]
- Hanlon P., Hanlon L., Marsh V., Byass P., Sillah H., Hayes R., Whittle H. C., Greenwood B. M. Serological comparisons of approaches to polio vaccination in the Gambia. Lancet. 1987 Apr 4;1(8536):800–801. doi: 10.1016/s0140-6736(87)92818-2. [DOI] [PubMed] [Google Scholar]
- Hull H. F., Ward N. A., Hull B. P., Milstien J. B., de Quadros C. Paralytic poliomyelitis: seasoned strategies, disappearing disease. Lancet. 1994 May 28;343(8909):1331–1337. doi: 10.1016/s0140-6736(94)92472-4. [DOI] [PubMed] [Google Scholar]
- Ismail H. I., Lal M. Poliomyelitis in Malaysia: two confirmed cases after 6 years without polio. Ann Trop Paediatr. 1993;13(4):339–343. doi: 10.1080/02724936.1993.11747668. [DOI] [PubMed] [Google Scholar]
- King G. E., Hadler S. C. Simultaneous administration of childhood vaccines: an important public health policy that is safe and efficacious. Pediatr Infect Dis J. 1994 May;13(5):394–407. [PubMed] [Google Scholar]
- Kok P. W., Leeuwenburg J., Tukei P., van Wezel A. L., Kapsenberg J. G., van Steenis G., Galazka A., Robertson S. E., Robinson D. Serological and virological assessment of oral and inactivated poliovirus vaccines in a rural population in Kenya. Bull World Health Organ. 1992;70(1):93–103. [PMC free article] [PubMed] [Google Scholar]
- Krishnan R., Jadhav M., John T. J. Efficacy of inactivated poliovirus vaccine in India. Bull World Health Organ. 1983;61(4):689–692. [PMC free article] [PubMed] [Google Scholar]
- Mas Lago P., Ramon Bravo J., Andrus J. K., Comellas M. M., Galindo M. A., de Quadros C. A., Bell E. Lessons from Cuba: mass campaign administration of trivalent oral poliovirus vaccine and seroprevalence of poliovirus neutralizing antibodies. Bull World Health Organ. 1994;72(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- McBean A. M., Thoms M. L., Albrecht P., Cuthie J. C., Bernier R. Serologic response to oral polio vaccine and enhanced-potency inactivated polio vaccines. Am J Epidemiol. 1988 Sep;128(3):615–628. doi: 10.1093/oxfordjournals.aje.a115009. [DOI] [PubMed] [Google Scholar]
- Moriniere B. J., van Loon F. P., Rhodes P. H., Klein-Zabban M. L., Frank-Senat B., Herrington J. E., Pallansch M. A., Patriarca P. A. Immunogenicity of a supplemental dose of oral versus inactivated poliovirus vaccine. Lancet. 1993 Jun 19;341(8860):1545–1550. doi: 10.1016/0140-6736(93)90693-b. [DOI] [PubMed] [Google Scholar]
- Pal S. R., Anerjee G., Aikat B. K. Serological investigation on endemicity of poliomyelitis in Calcutta and in a neighbouring rural area. Indian J Med Res. 1966 Jun;54(6):507–511. [PubMed] [Google Scholar]
- Patriarca P. A., Foege W. H., Swartz T. A. Progress in polio eradication. Lancet. 1993 Dec 11;342(8885):1461–1464. doi: 10.1016/0140-6736(93)92936-n. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Laender F., Palmeira G., Oliveira M. J., Lima Filho J., Dantes M. C., Cordeiro M. T., Risi J. B., Jr, Orenstein W. A. Randomised trial of alternative formulations of oral poliovaccine in Brazil. Lancet. 1988 Feb 27;1(8583):429–433. doi: 10.1016/s0140-6736(88)91229-9. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Wright P. F., John T. J. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991 Sep-Oct;13(5):926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- Ramia S., Arif M. Paralytic poliomyelitis outbreak in Gizan, Saudi Arabia. J Trop Pediatr. 1991 Aug;37(4):202–204. doi: 10.1093/tropej/37.4.202. [DOI] [PubMed] [Google Scholar]
- Robertson S. E., Suleiman A. J., Mehta F. R., al-Dhahry S. H., el-Bualy M. S. Poliomyelitis in Oman: acute flaccid paralysis surveillance leading to early detection and rapid response to a type 3 outbreak. Bull World Health Organ. 1994;72(6):907–914. [PMC free article] [PubMed] [Google Scholar]
- Simasathien S., Migasena S., Beuvery C., van Steenis G., Samakoses R., Pitisuttitham P., Vesikari T. Comparison of enhanced potency inactivated poliovirus vaccine (EIPV) versus standard oral poliovirus vaccine (OPV) in Thai infants. Scand J Infect Dis. 1994;26(6):731–738. doi: 10.3109/00365549409008643. [DOI] [PubMed] [Google Scholar]
- Simoes E. A., Padmini B., Steinhoff M. C., Jadhav M., John T. J. Antibody response of infants to two doses of inactivated poliovirus vaccine of enhanced potency. Am J Dis Child. 1985 Oct;139(10):977–980. doi: 10.1001/archpedi.1985.02140120023021. [DOI] [PubMed] [Google Scholar]
- Slater P. E., Orenstein W. A., Morag A., Avni A., Handsher R., Green M. S., Costin C., Yarrow A., Rishpon S., Havkin O. Poliomyelitis outbreak in Israel in 1988: a report with two commentaries. Lancet. 1990 May 19;335(8699):1192–1198. doi: 10.1016/0140-6736(90)92705-m. [DOI] [PubMed] [Google Scholar]
- Sutter R. W., Patriarca P. A., Brogan S., Malankar P. G., Pallansch M. A., Kew O. M., Bass A. G., Cochi S. L., Alexander J. P., Hall D. B. Outbreak of paralytic poliomyelitis in Oman: evidence for widespread transmission among fully vaccinated children. Lancet. 1991 Sep 21;338(8769):715–720. doi: 10.1016/0140-6736(91)91442-w. [DOI] [PubMed] [Google Scholar]
- Sutter R. W., Patriarca P. A., Suleiman A. J., Pallansch M. A., Zell E. R., Malankar P. G., Brogan S., al-Ghassani A. A., el-Bualy M. S. Paralytic poliomyelitis in Oman: association between regional differences in attack rate and variations in antibody responses to oral poliovirus vaccine. Int J Epidemiol. 1993 Oct;22(5):936–944. doi: 10.1093/ije/22.5.936. [DOI] [PubMed] [Google Scholar]
- Wood D. J., Heath A. B. The Second International Standard for anti-poliovirus sera types 1, 2 and 3. Biologicals. 1992 Sep;20(3):203–211. doi: 10.1016/s1045-1056(05)80039-9. [DOI] [PubMed] [Google Scholar]
- Yang C. F., De L., Holloway B. P., Pallansch M. A., Kew O. M. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 1991 Jul;20(2):159–179. doi: 10.1016/0168-1702(91)90107-7. [DOI] [PubMed] [Google Scholar]
- Zevering Y., Houghten R. A., Frazer I. H., Good M. F. Major population differences in T cell response to a malaria sporozoite vaccine candidate. Int Immunol. 1990;2(10):945–955. doi: 10.1093/intimm/2.10.945. [DOI] [PubMed] [Google Scholar]
- Zevering Y., Khamboonruang C., Rungruengthanakit K., Tungviboonchai L., Ruengpipattanapan J., Bathurst I., Barr P., Good M. F. Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6118–6122. doi: 10.1073/pnas.91.13.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quadros C. A., Andrus J. K., Olive J. M., Guerra de Macedo C., Henderson D. A. Polio eradication from the Western Hemisphere. Annu Rev Public Health. 1992;13:239–252. doi: 10.1146/annurev.pu.13.050192.001323. [DOI] [PubMed] [Google Scholar]
- van Niekerk A. B., Vries J. B., Baard J., Schoub B. D., Chezzi C., Blackburn N. K. Outbreak of paralytic poliomyelitis in Namibia. Lancet. 1994 Sep 3;344(8923):661–664. doi: 10.1016/s0140-6736(94)92090-7. [DOI] [PubMed] [Google Scholar]


