Abstract
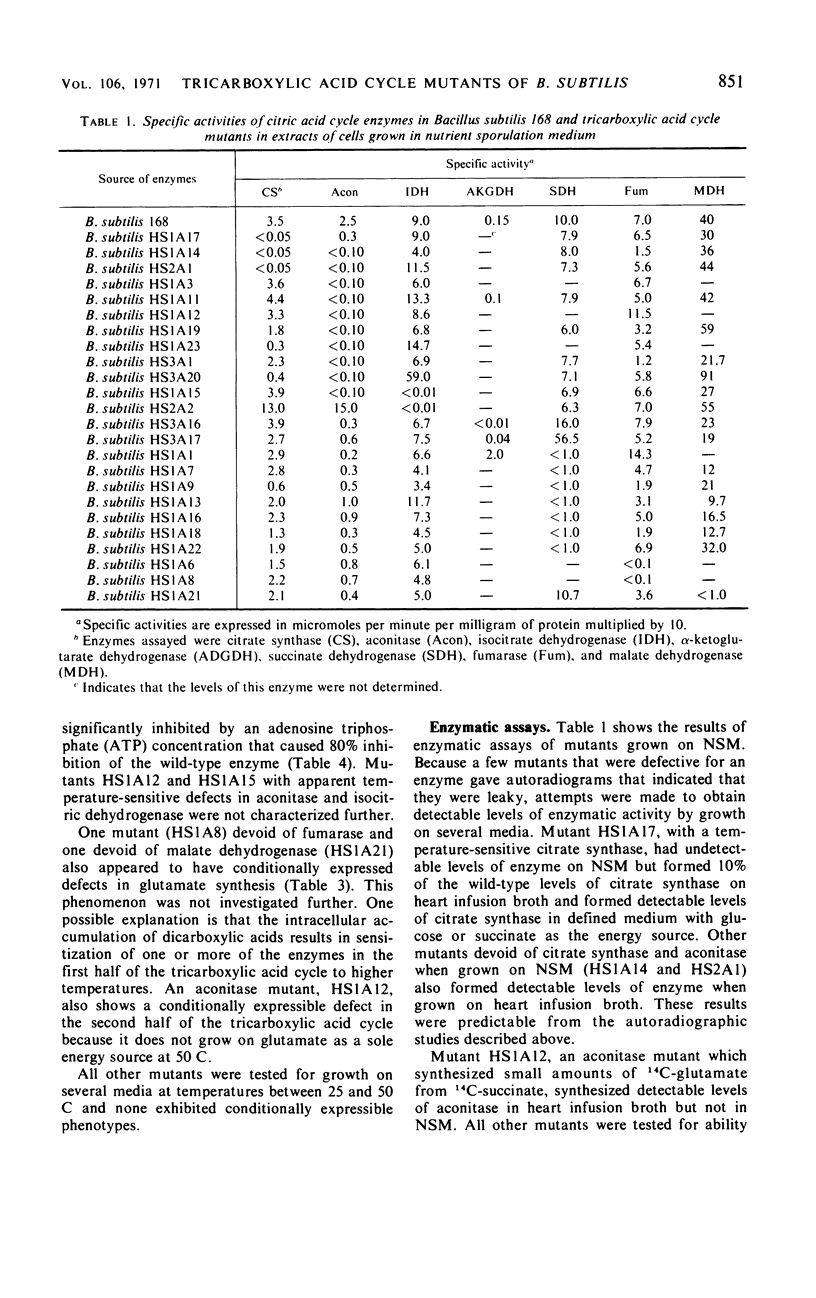
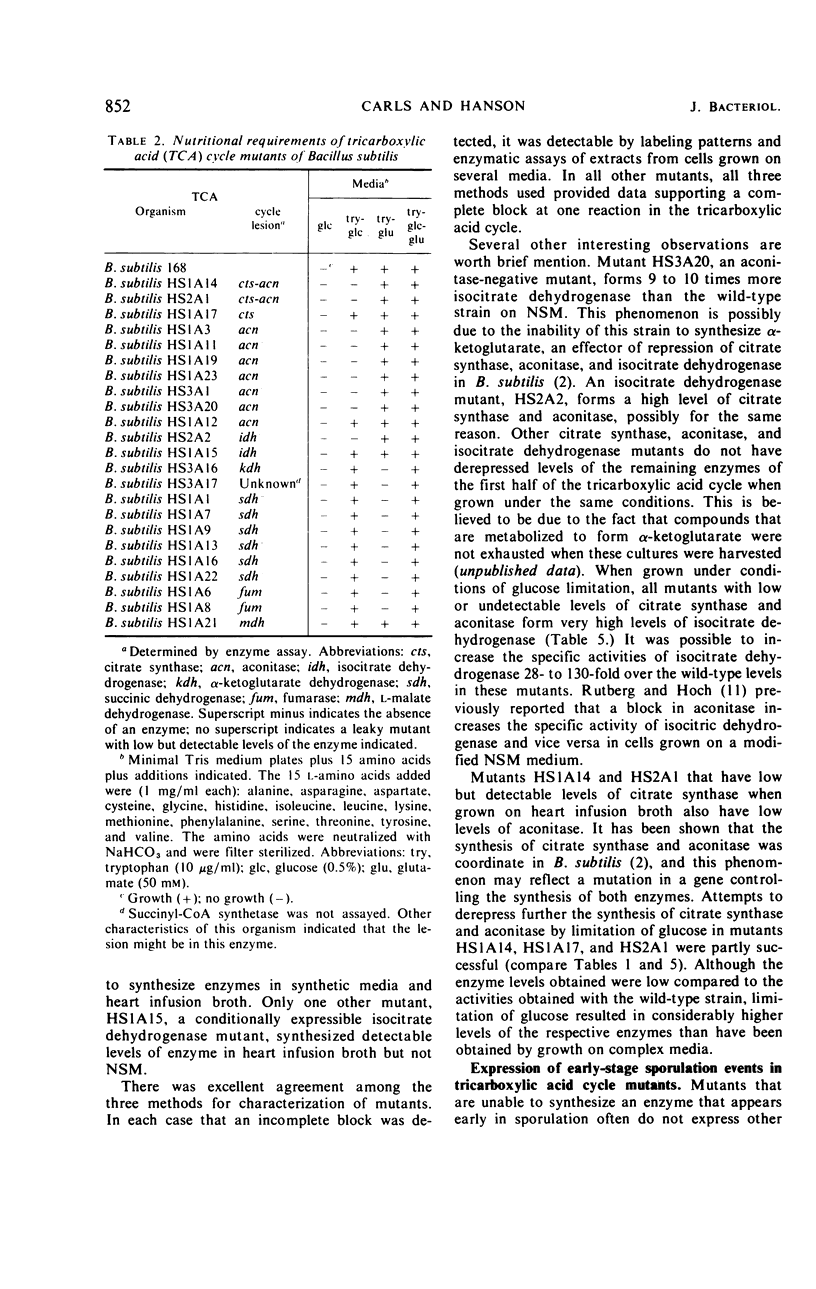
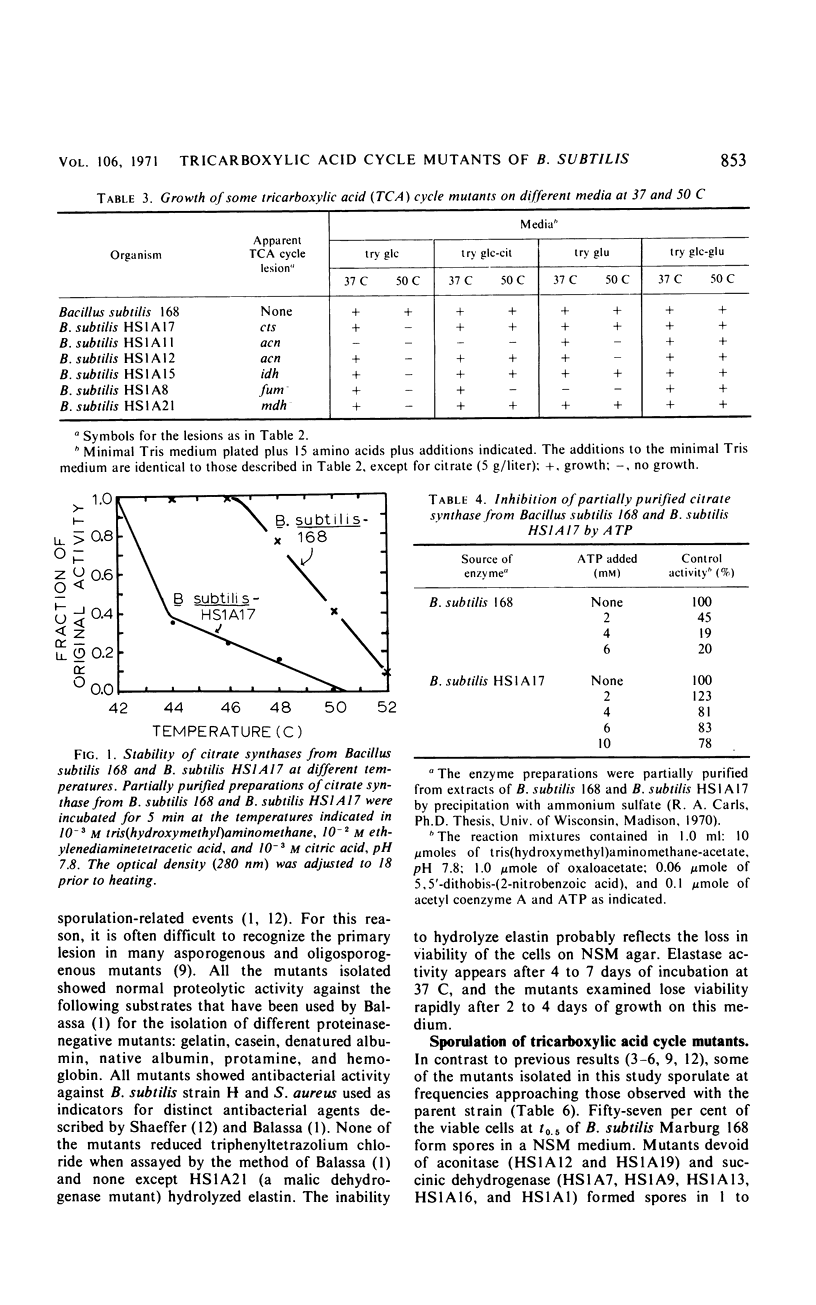
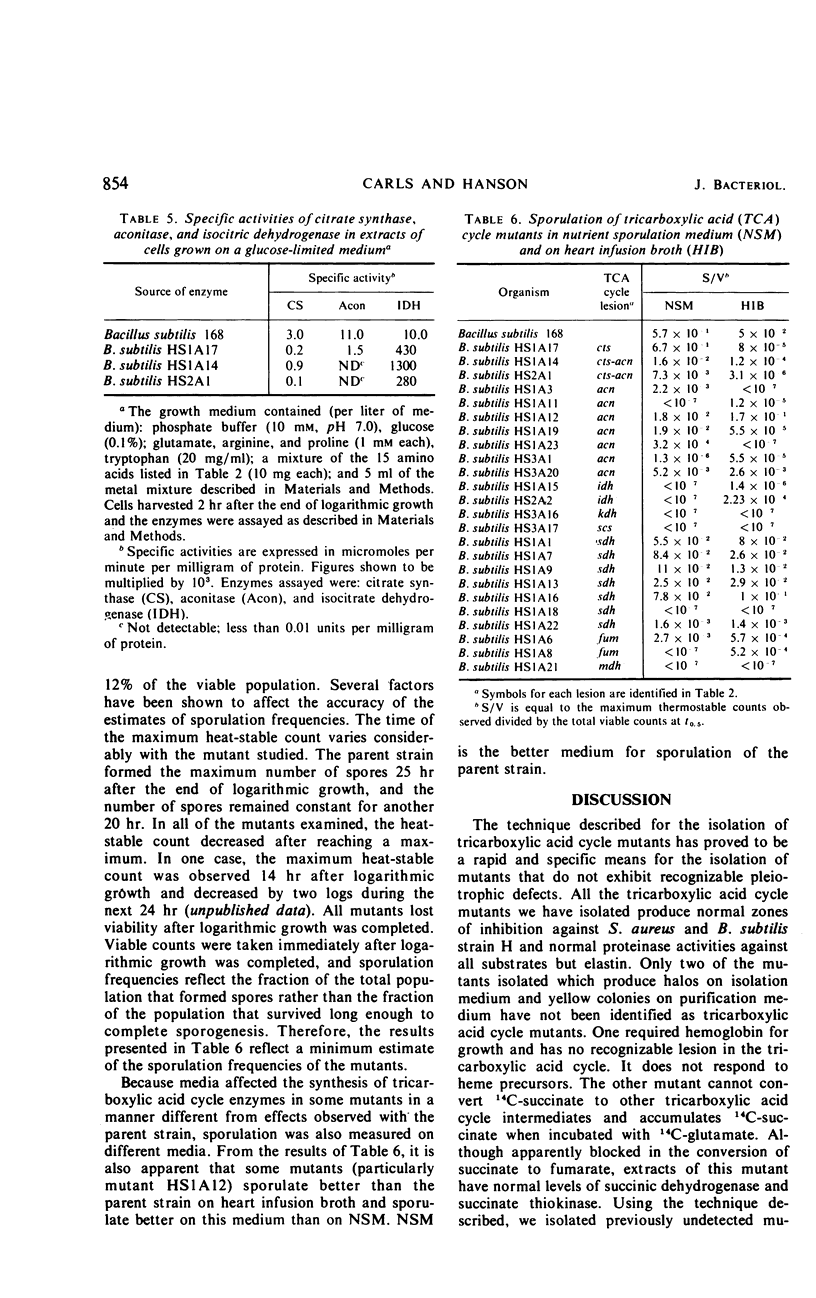
A technique was developed for the detection, on agar, of mutants of Bacillus subtilis that lacked a functional tricarboxylic acid cycle. Mutants devoid of detectable levels of aconitase, isocitric dehydrogenase, alpha-ketoglutarate dehydrogenase, succinic dehydrogenase, fumarase, and malate dehydrogenase have been isolated and characterized. Several mutants with conditionally expressible lesions, including a mutant with a heat-sensitive citrate synthase, have also been isolated. All of the mutants examined express all the biochemical markers normally absent in early-stage sporulation mutants except elastase, and some of these mutants sporulated nearly as well as the prototroph.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balassa G. Biochemical genetics of bacterial sporulation. I. Unidirectional pleiotropic interactions among genes controlling sporulation in Bacillus subtilis. Mol Gen Genet. 1969;104(1):73–103. [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Coarse and fine control of citrate synthase from Bacillus subtilis. Biochim Biophys Acta. 1969 Jul 30;184(2):252–262. doi: 10.1016/0304-4165(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Peterson J. A., Yousten A. A. Unique biochemical events in bacterial sporulation. Annu Rev Microbiol. 1970;24:53–90. doi: 10.1146/annurev.mi.24.100170.000413. [DOI] [PubMed] [Google Scholar]
- Northrop J., Slepecky R. A. Sporulation mutations induced by heat in Bacillus subtilis. Science. 1967 Feb 17;155(3764):838–839. doi: 10.1126/science.155.3764.838. [DOI] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


