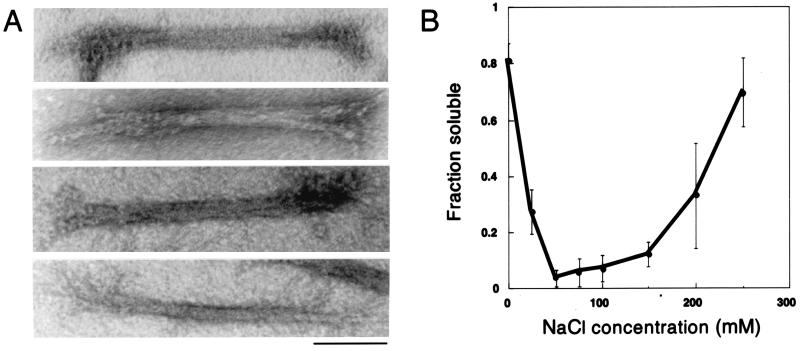
Figure 3.
GFP-RLC-myosin rod has filament formation characteristics similar to those of wild-type myosin II. (A) Negatively stained bipolar thick filaments of GFP-RLC-myosin rod formed in a buffer containing 50 mM NaCl and 10 mM MgCl2. (Bar, 0.1 μm.) (B) Purified proteins were dissolved in buffer containing NaCl at various concentrations. Centrifugation separated the solubilized protein, which remained in the supernatant, from the higher-order assemblies in the pellet. GFP-RLC-myosin rod has salt-dependent assembly characteristics similar to those of wild-type myosin II assayed under the same conditions. The mean fraction soluble is plotted for each salt concentration, and the error bars represent the standard deviation from seven experiments.