Abstract
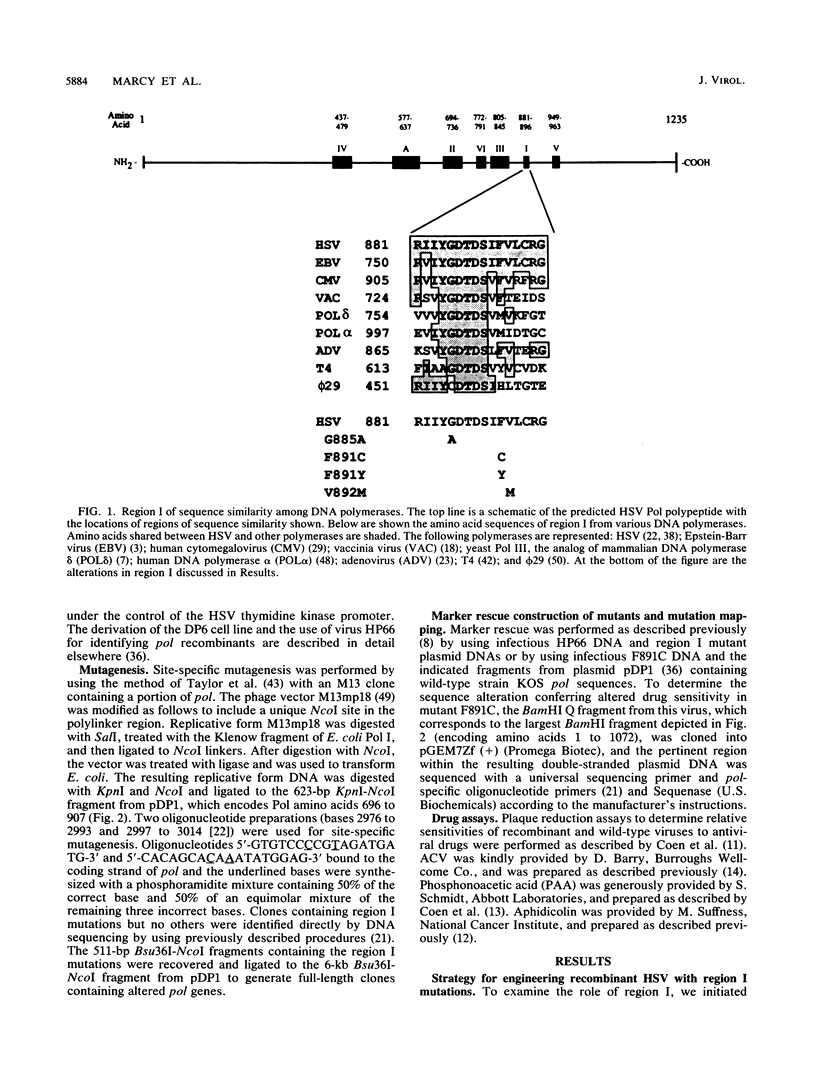
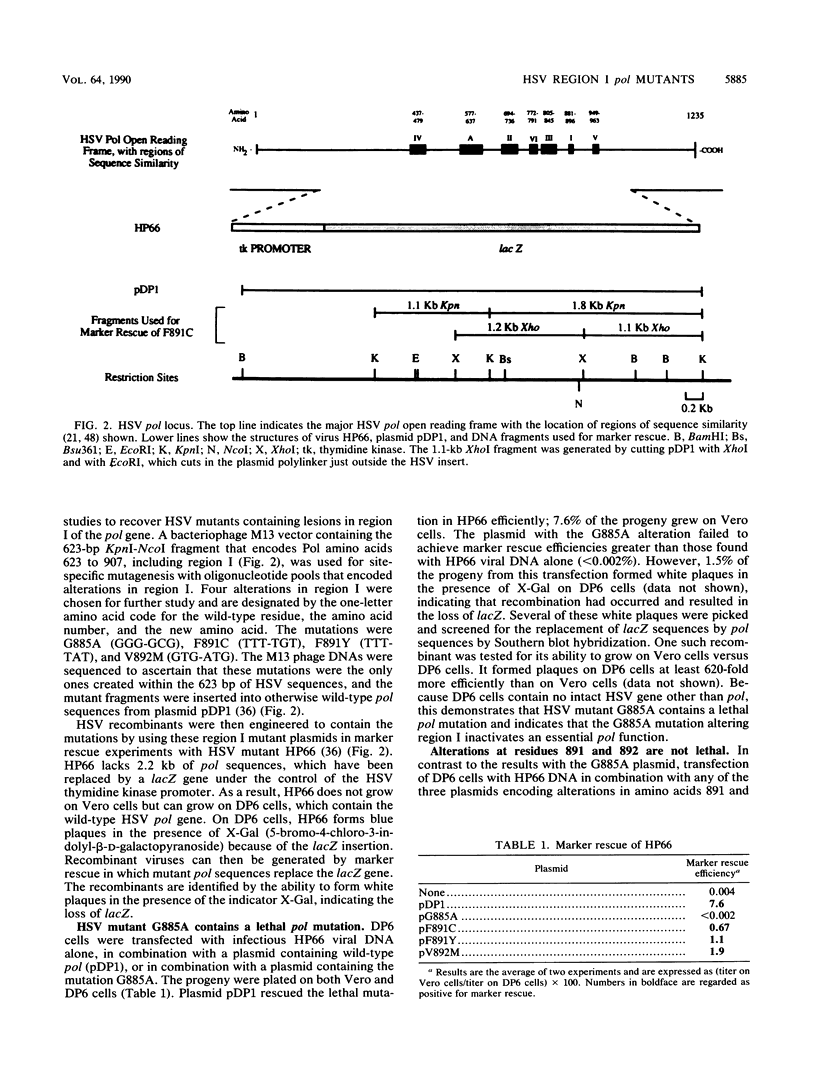
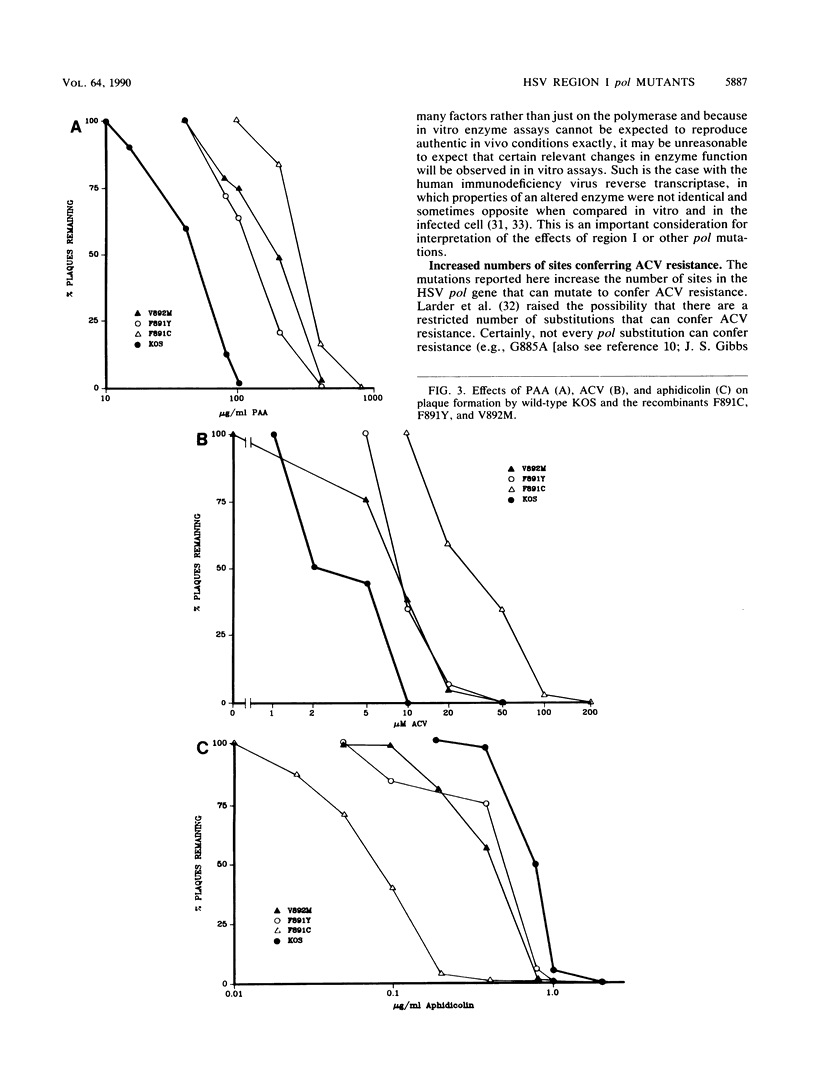
Eucaryotic, viral, and bacteriophage DNA polymerases of the alpha-like family share blocks of sequence similarity, the most conserved of which has been designated region I. Region I includes a YGDTDS motif that is almost invariant within the alpha-like family and that is similar to a motif conserved among RNA-directed polymerases and also includes adjacent amino acids that are more moderately conserved. To study the function of these conserved amino acids in vivo, site-specific mutagenesis was used to generate herpes simplex virus region I mutants. A recombinant virus constructed to contain a mutation within the nearly invariant YGDTDS motif was severely impaired for growth on Vero cells which do not contain a viral polymerase gene. However, three recombinants constructed to contain mutations altering more moderately conserved residues grew on Vero cells and exhibited altered sensitivities to nucleoside and PPi analogs and to aphidicolin. Marker rescue and DNA sequencing of one such recombinant demonstrated that the region I alteration confers the altered drug sensitivity phenotype. These results indicate that this region has an essential role in polymerase function in vivo and is involved directly or indirectly in drug and substrate recognition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Tucker A. D., Philipson L. Primary structural relationships may reflect similar DNA replication strategies. Virology. 1986 Mar;149(2):208–216. doi: 10.1016/0042-6822(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Becker Y. Computer-assisted primary and secondary structure analyses of DNA polymerases of herpes simplex, Epstein-Barr and varicella zoster viruses reveal conserved domains with some homology to DNA-binding domain in E. coli DNA pol I. Virus Genes. 1988 Jul;1(4):351–367. doi: 10.1007/BF00257098. [DOI] [PubMed] [Google Scholar]
- Bernad A., Blanco L., Lázaro J. M., Martín G., Salas M. A conserved 3'----5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989 Oct 6;59(1):219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- Bernad A., Lázaro J. M., Salas M., Blanco L. The highly conserved amino acid sequence motif Tyr-Gly-Asp-Thr-Asp-Ser in alpha-like DNA polymerases is required by phage phi 29 DNA polymerase for protein-primed initiation and polymerization. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4610–4614. doi: 10.1073/pnas.87.12.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet A., Simon M., Faye G., Bauer G. A., Burgers P. M. Structure and function of the Saccharomyces cerevisiae CDC2 gene encoding the large subunit of DNA polymerase III. EMBO J. 1989 Jun;8(6):1849–1854. doi: 10.1002/j.1460-2075.1989.tb03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou H. C., Weller S. K., Coen D. M. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology. 1985 Sep;145(2):213–226. doi: 10.1016/0042-6822(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Aschman D. P., Gelep P. T., Retondo M. J., Weller S. K., Schaffer P. A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984 Jan;49(1):236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Fleming H. E., Jr, Leslie L. K., Retondo M. J. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J Virol. 1985 Feb;53(2):477–488. doi: 10.1128/jvi.53.2.477-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Larder B. A., Oliver N. M., Kemp S., Smith I. W., Darby G. Characterization of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J Gen Virol. 1989 Feb;70(Pt 2):375–382. doi: 10.1099/0022-1317-70-2-375. [DOI] [PubMed] [Google Scholar]
- Dicioccio R. A., Chadha K., Sahai Srivastava B. I. Inhibition of herpes simplex virus-induced DNA polymerase, cellular DNA polymerase alpha, and virus production by aphidicolin. Biochim Biophys Acta. 1980 Sep 19;609(2):224–231. doi: 10.1016/0005-2787(80)90233-6. [DOI] [PubMed] [Google Scholar]
- Dorsky D. I., Crumpacker C. S. Site-specific mutagenesis of a highly conserved region of the herpes simplex virus type 1 DNA polymerase gene. J Virol. 1990 Mar;64(3):1394–1397. doi: 10.1128/jvi.64.3.1394-1397.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Jones E. V., Moss B. Homology between DNA polymerases of poxviruses, herpesviruses, and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Derse D. D., Bastow K. F., Cheng Y. C. Novel interaction of aphidicolin with herpes simplex virus DNA polymerase and polymerase-associated exonuclease. J Biol Chem. 1984 Nov 10;259(21):13282–13286. [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Bastow K. F., Cheng Y. C., Coen D. M. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6672–6676. doi: 10.1073/pnas.85.18.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Hall J. D., Mount D. W., Retondo M. J., Weller S. K., Coen D. M. Sequence and mapping analyses of the herpes simplex virus DNA polymerase gene predict a C-terminal substrate binding domain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7969–7973. doi: 10.1073/pnas.82.23.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Hall J. D., Wang Y. S., Pierpont J., Berlin M. S., Rundlett S. E., Woodward S. Aphidicolin resistance in herpes simplex virus type I reveals features of the DNA polymerase dNTP binding site. Nucleic Acids Res. 1989 Nov 25;17(22):9231–9244. doi: 10.1093/nar/17.22.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman T. C. An amino acid sequence motif linking viral DNA polymerases and plant virus proteins involved in RNA replication. Nucleic Acids Res. 1986 Aug 26;14(16):6769–6769. doi: 10.1093/nar/14.16.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi Y., Hirashima A. Interference with viral infection by defective RNA replicase. J Virol. 1987 Dec;61(12):3946–3949. doi: 10.1128/jvi.61.12.3946-3949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf C. W. Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 1 strain Angelotti. Nucleic Acids Res. 1986 Oct 24;14(20):8225–8226. doi: 10.1093/nar/14.20.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf C. W., Weisshart K. The herpes simplex virus DNA polymerase: analysis of the functional domains. Biochim Biophys Acta. 1988 Dec 20;951(2-3):298–314. doi: 10.1016/0167-4781(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Purifoy D. J. Infectious potential of human immunodeficiency virus type 1 reverse transcriptase mutants with altered inhibitor sensitivity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4803–4807. doi: 10.1073/pnas.86.13.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy A. I., Yager D. R., Coen D. M. Isolation and characterization of herpes simplex virus mutants containing engineered mutations at the DNA polymerase locus. J Virol. 1990 May;64(5):2208–2216. doi: 10.1128/jvi.64.5.2208-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reha-Krantz L. J. Locations of amino acid substitutions in bacteriophage T4 tsL56 DNA polymerase predict an N-terminal exonuclease domain. J Virol. 1989 Nov;63(11):4762–4766. doi: 10.1128/jvi.63.11.4762-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno J. M., Lee L. F., Boezi J. A. Inhibition of herpesvirus replication and herpesvirus-induced deoxyribonucleic acid polymerase by phosphonoformate. Antimicrob Agents Chemother. 1978 Feb;13(2):188–192. doi: 10.1128/aac.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks S. L., Wanklin R. J., Reece D. E., Hicks K. A., Tyler K. L., Coen D. M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989 Dec 1;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- Spicer E. K., Rush J., Fung C., Reha-Krantz L. J., Karam J. D., Konigsberg W. H. Primary structure of T4 DNA polymerase. Evolutionary relatedness to eucaryotic and other procaryotic DNA polymerases. J Biol Chem. 1988 Jun 5;263(16):7478–7486. [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi T., Maeno K., Nishiyama Y. A single-base change within the DNA polymerase locus of herpes simplex virus type 2 can confer resistance to aphidicolin. J Virol. 1987 Feb;61(2):388–394. doi: 10.1128/jvi.61.2.388-394.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S., Wong S. W., Korn D. Human DNA polymerase alpha: predicted functional domains and relationships with viral DNA polymerases. FASEB J. 1989 Jan;3(1):14–21. doi: 10.1096/fasebj.3.1.2642867. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Nucleotide sequence of the major early region of bacteriophage phi 29. Gene. 1982 Mar;17(3):323–335. doi: 10.1016/0378-1119(82)90149-4. [DOI] [PubMed] [Google Scholar]


