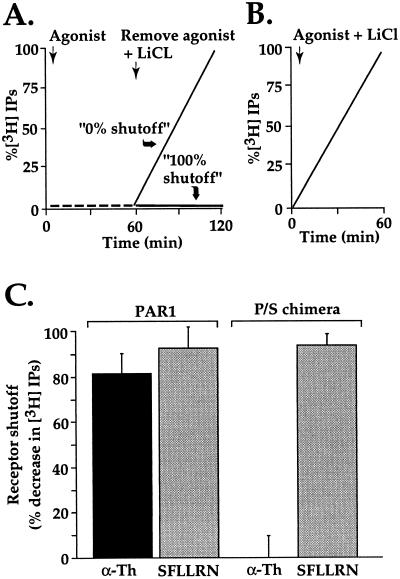
Figure 1.
Persistent signaling by P/S chimera after activation by thrombin but not SFLLRN. (A) Schematic of experimental design. Cells labeled with myo-[3H]inositol were incubated with agonist in the absence of LiCl; under these conditions, phosphoinositide hydrolysis is stimulated but IPs do not accumulate. After 60 min, agonist was removed and LiCl was added to allow accumulation of IPs during a subsequent incubation period as a measure of persistent signaling in the absence of agonist. Results were normalized to the amount of IPs accumulated when agonist and LiCl were added simultaneously (B) and expressed as percent shutoff (i.e., zero shutoff = no difference in IP accumulation under conditions A vs. B.) (C) Phosphoinositide hydrolysis was measured in PAR1-deficient mouse lung fibroblasts stably expressing similar amounts of surface wild-type PAR1 or the P/S chimera. myo-[3H]Inositol labeled cells were incubated with 100 μM SFLLRN or 10 nM α-Th for 60 min at 25°C in the absence of LiCl. Agonist was removed and cells were washed three times with DMEM containing 0.5 units/ml hirudin (α-Th inhibitor). Cells were then incubated with DMEM containing 20 mM LiCl and 0.5 units/ml hirudin at 25°C for an additional 60 min, at which time accumulated [3H]IPs were measured. The data are the mean % shutoff (see above) ± SD (n = 3). In PAR1-expressing cells, α-Th and SFLLRN caused an initial 3- and 3.8-fold increase in phosphoinositide hydrolysis, respectively; whereas cells expressing P/S chimera showed an initial 13- and 16-fold increase in PI hydrolysis to α-Th and SFLLRN, respectively. Similar results were obtained in three separate experiments. Note remarkable failure of α-Th-activated P/S chimera to shut off.